We retrospectively examined surgical outcomes for Vesicovaginal Fistulas (VVF) repaired through an abdominal, transperitoneal approach. Failure of VVF repair was defined as any urine leakage from the repaired fistula tract after hospital discharge.
Thirty-three abdominal VVF repairs were identified. Mean patient age was 46 years and mean fistula size was 2 cm. The most common etiology and location of fistula were hysterectomy (76%) and trigone (51%).
Over a mean of 12 months follow up, 25 of the fistulas (76%) were successfully closed after the initial attempt. Failure of VVF repair occurred more commonly in patients with new post-operative OAB symptoms (OR 7.3, p = 0.03). There were trends toward failure in patients using tobacco (OR 5.3, p = 0.06) and with preoperative comorbidities potentially affecting wound healing (OR 5.3, p = 0.06).
Abdominal VVF repair is an effective technique for VVF repair.
Complications, Fistula, Flap, Outcomes, Risk factors, Vesicovaginal fistula
Vesicovaginal fistula (VVF) affects an estimated three million women internationally. In the United States, VVF occurs most commonly after pelvic surgery and is also associated with trauma or pelvic radiation. It is a rare complication, with reported incidence of 0.05-2% after gynecologic procedures [1]. VVFs have a significant impact on a patient's quality of life and an adverse economic impact due to lost work hours. Surgical repair is the gold standard treatment for VVF and numerous closure techniques have been documented since Marion Sims first reported a successful closure in 1852. Successful closure rates for VVF repair vary considerably in the literature [2,3], likely due to differing patient demographics, complexity, and repair strategies.
Although an abdominal surgical approach is frequently cited as an option for VVF repair, there are few contemporary series examining surgical outcomes for this technique in a US patient population. Many published abdominal VVF series focus entirely on obstetric fistulas [4] or combine outcomes for vaginal and abdominal procedures [5,6]. Given the small sample sizes and potential heterogeneity among these existing series, limited data are available to counsel contemporary, nonobstetric VVF patients undergoing abdominal repairs about outcomes and risk factors for surgical failure.
Our goal was to examine surgical outcomes for abdominal VVF repairs in a contemporary referral population and investigate if any demographic, operative, or post-operative variables were associated with increased risk of repair failure.
Departmental administrative billing codes were used to identify all patients who underwent VVF repair at a single tertiary referral hospital between the years 1996-2012. IRB approval was obtained for the retrospective review. Patients with complete urethral destruction from catheter induced erosions were excluded from analysis. Abdominal VVF cases were selected and confirmed through review of operative notes. Electronic medical records were used to extract additional demographic, operative, and post-operative data from these patients. Outside records were utilized to determine initial presentation and fistula management. Assessed comorbidities were limited to factors thought to impact post-operative wound healing such as diabetes mellitus (defined as undergoing oral or subcutaneous treatment for elevated blood sugar), prior abdominal/pelvic radiation, and chronic urinary tract infections (> 3 treated infections/year prior to fistula repair). These comorbidities could not be assessed separately due to small sample size and were thus analyzed as an aggregate preoperative co-morbidity variable. Smoking, which may also potentially affect wound healing, was analyzed independently as a risk factor due to larger sample size. The smoking variable was considered positive if the patient was using tobacco within 1 month of the abdominal VVF repair.
An abdominal VVF repair was utilized for a fistula with multiple tracts, previous failed VVF repair, and/or if inaccessible via vaginal approach (Figure 1). Repairs were approached through an infraumbilical, midline incision and a transperitoneal VVF repair technique was used for all patients. To briefly summarize the technique, cystoscopy was first performed and the fistula tract was intubated with a wire or ureteral catheter. If the fistula was located in close proximity to the ureteral orifices, ureters were also commonly stented so ureteral orifices are not damaged during repair. Lysis of adhesions was then performed to separate abdominal viscera from the posterior peritoneum covering the bladder. Once adequate exposure was obtained, the retropubic space was entered and the bladder was mobilized from the pelvic side walls, leaving the bladder vascular pedicles intact. The peritoneum was next separated from the posterior wall of the bladder down to the vaginal apex. Starting approximately 5 cm above the fistula, the bladder was then bisected along the posterior wall to the fistula tract, as identified by the catheter(s). The edges of the now visible fistula tract were excised from both the bladder and the vagina, back to viable tissue. After resecting the fistula tract, the surgical plain between bladder and vagina was sharply dissected around the excised tract for an additional 2 cm margin. Once adequate tissue was mobilized, the vagina and bladder were then closed independently with at least 2 non-overlapping absorbable suture lines (Figure 2). An interposition flap of peritoneum or omentum was placed between bladder and vaginal closures at the discretion of the treating surgeon.
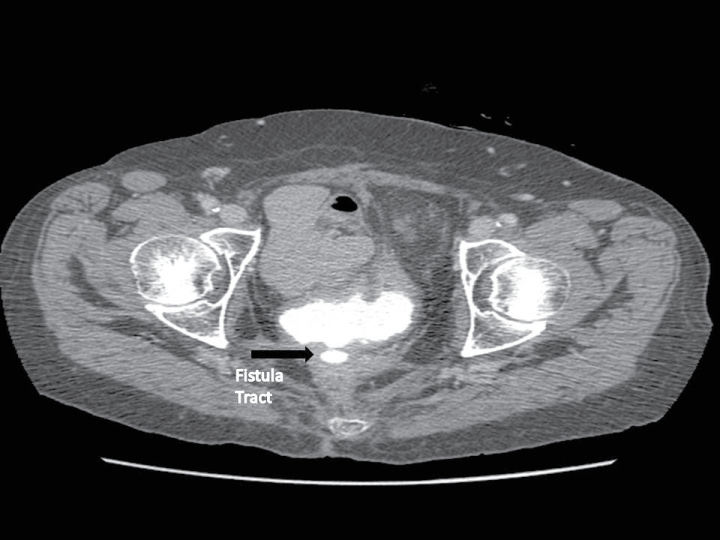 Figure 1: Example of type of VVF selected for abdominal repair. Patient had developed a VVF after low anterior resection for rectal cancer and pelvic radiation. View Figure 1
Figure 1: Example of type of VVF selected for abdominal repair. Patient had developed a VVF after low anterior resection for rectal cancer and pelvic radiation. View Figure 1
 Figure 2: A) VVF located between bladder and vagina with posterior peritoneum covering; B) Posterior peritoneum mobilized, bladder and vagina separated, fistula tract excised and opening in bladder and vagina independently closed. View Figure 2
Figure 2: A) VVF located between bladder and vagina with posterior peritoneum covering; B) Posterior peritoneum mobilized, bladder and vagina separated, fistula tract excised and opening in bladder and vagina independently closed. View Figure 2
Fistula data, including location and size, were determined through review of operative documentation at time of repair. Operative length and estimated blood loss were extracted from anesthesia records. For this analysis, use of either omentum or vascularized peritoneum placed between bladder and vaginal suture lines was characterized as an interposition flap.
All patients had initial post-operative follow-up 1-3 weeks after surgery or earlier for any new symptoms of urinary incontinence, overactive bladder symptoms, or dysuria. Patients had additional scheduled follow up visits 3-6 months after repair but may have had these visits with local urologist's due to travel restrictions. Phone records and scanned outside documents were reviewed to identify any post-operative symptoms or admissions to outside hospitals. A patient was classified as having new, persistent overactive bladder symptoms post-operatively after VVF repair if she had documentation of any treatment or assessment for new, daily symptoms of urinary urgency, urge incontinence, or inability to delay urination.
The primary outcome for this study was failure of abdominal VVF repair. Patients were examined during post-operative follow up with physical exam, cystoscopy and/or cystogram to evaluate the integrity of the repair. Surgical failure of VVF surgery was defined as any confirmed urine leakage from the repaired fistula tract after hospital discharge. This leakage was distinguished from post-operative urinary incontinence by physical exam and confirmed with a negative radiologic and cystoscopic workup. Data from the last documented visit or communication were used to determine repair status and length of follow-up. Data were collected by a third party who did not participate in any of the repairs or post-operative care.
Continuous variables were compared with t-test/ANOVA and presented as mean (90% CI). Categorical variables were compared with Fisher exact tests and strength of associations presented with Odd's ratios. Analysis using SAS (Cary, NC) statistical software was performed to identify variables significantly associated (p < 0.05) with post-operative VVF failure.
Between 1996 and 2012, 112 patients underwent VVF repair and 33 (29%) of these patients had an abdominal VVF repair, as identified through departmental billing codes and operative notes. Mean patient age at time of VVF surgery was 46 years (90% CI 42 - 50), mean fistula size was 2.0 cm (1.4 - 2.6) and the most common fistula location was trigonal (51%). Twenty-five (76%), 7 (21%), and 1 (3%) of the fistulas were attributed to previous hysterectomy surgery, complications from radiation/abdominal surgery, and caesarean section, respectively. Twelve patients in this cohort had at least one previous failed attempt at VVF closure. Mean time with fistula before abdominal VVF repair was 8 months (6 - 10) per data available from 25 patients.
No cases were aborted and all VVF were closed at the end of the procedure, per operative notes. Mean length of surgery was 246 minutes (192 - 300, 21 cases) and 56% of the closures used an interposition flap. Catheter drainage was maintained for a mean 19 days (17 - 21) after surgery.
Over a mean follow up of 12 months (3 - 22), 76% (25/33) of the fistulas were successfully closed after the initial attempt. Two patients had significant post-operative complications, including small bowel obstruction 6 months after abdominal VVF repair requiring operative repair and an enterovaginal fistula 6 months after repair. Failure of VVF repair was more commonly associated with new post-operative OAB symptoms (OR 7.7, p = 0.03). There were also trends toward increased odds of failure in patients with a positive tobacco history prior to repair (OR 5.3, p = 0.06) and with preoperative comorbidities potentially affecting wound healing (OR 5.3, p = 0.06). Previous attempts at closure, fistula size, etiology of fistula (surgical vs radiation), patient age, operative time, lack of interposition flap, or length post-operative catheterization were not associated with failure in this cohort. Table 1 summarizes additional demographic, operative, post-operative analysis for associations with VVF failure.
Table 1: Demographics of successful vs. failed abdominal VVF repairs. View Table 1
For the 8 failed repairs, subsequent surgery was undertaken less than 3 months after initial surgery in 2 patients, between 3 - 12 months in 4 patients, and greater than 12 months in 1 patient. Six VVFs remained closed after second attempt at closure and 1 fistula required a third repair. All seven of these patients reported successful fistula closure at last follow up. One patient is still awaiting diversion for enterovaginal fistula.
The abdominal vesicovaginal fistula surgical technique used for this nonobstetric VVF cohort successfully closed 76% of presenting fistulas. The variable most strongly associated with repair failure was new, persistent OAB symptoms requiring assessment or intervention. There were also trends suggesting that failure was also associated with using tobacco prior to repair or having comorbidities potentially affecting wound healing (abdominal radiation/diabetes/chronic UTI). Failure of abdominal VVF repair was not associated with variables commonly perceived to be failure risk factors, such as larger fistulas or lack of interposition flaps.
Our outcomes compare similarly to the 75% initial success rate reported by Ockim, et al. in a series of 24 abdominal VVF repairs [7], but lower than those reported by Hadzi-Djokic (32 patients, 94% success) [8], and Rahjamaheswari (19 patients, 100%) [6]. It is challenging to compare outcomes between patient cohort's due to potentially differing demographics, definition of successful closure, and follow up. Although fistula sizes from these few contemporary series were similar to our cohort, demographics from the limited number of abdominal repairs were not independently presented. For example, smoking status, diabetes, and exposure to previous pelvic radiation is not frequently reported in many series. By presenting expanded demographics from our cohort, we hope to place our outcomes in a context such that future outcome comparisons between series will be more rigorous. Ultimately, a standardized VVF outcome reporting system should be developed to better understand outcome differences in technique and patient populations.
We identified trends toward increased failure of abdominal VVF repairs in patients using tobacco prior to repair and in patients with any combination of diabetes/chronic UTI/radiation comorbidities. Our study likely underestimates the impact of tobacco variable since it was difficult to differentiate volume or length of tobacco usage through retrospective review. We suggest that these specific variables were associated with a higher post-operative risk of failure because each can potentially impair wound healing and pose a greater risk of post-operative tissue breakdown. Wound healing impairment can occur with diabetic patients, chronic inflammation and radiation exposure. Given the potentially long suture lines and larger repair areas affected during abdominal VVF repairs, it is reasonable to suggest that risk factors affecting wound healing may affect fistula closure outcomes.
New onset, persistent overactive bladder symptoms after VVF repair in this study are also associated with a significantly increased risk of failure. We suggest that new, persistent OAB symptoms after VVF repair could reflect inflammation, edema, tension, or bleeding in the repair site and thus affect wound healing. Normally, irritative symptoms tend to decrease after VVF repair as the bladder heals. Dolan, et al. assessed 31 patients with successful VVF repair and 87% reported that their urinary symptoms had little negative impact on quality of life at a mean 50 months after surgery [9]. Since it is the expectation that bladder symptoms should improve over time after VVF repair, we suggest that a patient with new progressive OAB symptoms after abdominal VVF repair may need special assessment for potential fistula repair breakdown. Due to limitations of this retrospective study, we do not have information regarding onset of fistula related to time of VVF repair. In general, it is our practice to wait at least 3 months from time of fistula presentation before attempting repair. We also delay repair until it appears that the fistula edges are mature and organized on physical cystoscopic exam. Investigating time from onset as variable may also give more insight into risk of developing new OAB symptoms after VVF repair and how timing of repair affects these findings.
It is interesting to note that lack of an interposition flap was not associated with increased risk for VVF failure in our series. There is controversy regarding the use of interposition flaps during VVF repair. Pshak, et al. did not note increased failure in their series of 73 vaginal VVF patients repaired without interposition flaps [10]. In contrast, a retrospective evaluation of 41 VVF/UVF at a tertiary center in the United Kingdom found that complex abdominal fistula repairs had a higher risk failure if omental flap interposition was not used [7]. Additionally, in a contemporary series of abdominal and vaginal VVF repair, Lee, et al. reported that an interposition flap was used in all 19 of their abdominal VVF repair to good outcomes [5]. A randomized trial is needed to better assess which VVF characteristics and surgical approaches benefit most from concomitant interposition use.
We acknowledge the limitations present in this retrospective review. First, the patient cohort spanned multiple years (1996-2012), making it difficult to determine whether surgeon experience or evolving surgical techniques had any impact on failure. However, review of operative notes revealed a fairly standard surgical template for repairs over the study time frame. Second, we recognize that failure in this population was likely multifactorial. The relatively small cohort size limited investigation on how variables potentially interacted. Finally, we recognize that our study may have under-estimated the failure rate for the population. The cohort represents a large clinical referral region, with average travel distance of almost 100 miles, and some patients may have sought follow-up elsewhere if failure occurred. However, phone and outside records suggest patients had access to a good post-operative communication system during follow up.
Despite limitations, this study presents dedicated outcomes for abdominal vesicovaginal fistula repair in a contemporary, non-obstetric VVFs cohort. Findings from this study highlight some risk factors associated with failure in our population which urologists/urogynecologists may wish to examine in their specific patient populations before an abdominal repair is performed (smoking status, diabetes) and follow after repair is completed (new post-operative OAB symptoms). More prospective research is needed to better determine common risk factors for failure across a larger VVF population.
Abdominal VVF repair of complex non-obstetric fistulas is a successful technique for vesicovaginal fistulas that are large, have multiple tracts or cannot be accessed via a vaginal approach. New post-operative OAB symptoms were associated with initial VVF repair failure.