International Journal of Cancer and Clinical Research
Long-Term Survival and Quality of Life Outcomes with Multiple Gamma Knife Radiosurgeries for Metastatic Breast Cancer to the Brain: Case Report and Review of Literature
Andrew T Roehrig1,3, Ethan Ferrel1,3, Robert K Fairbanks1,2, Wayne T Lamoreaux1,2, Alexander R MacKay1,2, Jason A Call2, John J Demakas1,4, Aaron Wagner1,2, Barton S Cooke1, Benjamin C. Ling1,5, Jonathan D. Carlson1,5 and Christopher M Lee1,2*
1Gamma Knife of Spokane, Spokane, WA, USA
2Cancer Care Northwest, Spokane, WA, USA
3University of Washington School of Medicine, Seattle, WA, USA
4Spokane Brain & Spine, Spokane, WA, USA
5Inland Neurosurgery & Spine, Spokane, WA, USA
*Corresponding author:
Christopher M. Lee, Gamma Knife of Spokane, Cancer Care Northwest, 910 W 5th Ave, Suite 102, Spokane, WA 99204, USA, Tel: 509-228-1000, Fax: 509-228-1183, E-mail: lee@ccnw.net
Int J Cancer Clin Res, IJCCR-2-036, (Volume 2, Issue 5), Case Report; ISSN: 2378-3419
Received: August 27, 2015 | Accepted: December 12, 2015 | Published: December 14, 2015
Citation: Roehrig AT, Ferrel E, Fairbanks RK, Lamoreaux WT, MacKay AR, et al. (2015) Long-Term Survival and Quality of Life Outcomes with Multiple Gamma Knife Radiosurgeries for Metastatic Breast Cancer to the Brain: Case Report and Review of Literature. Int J Cancer Clin Res 2:036. 10.23937/2378-3419/2/5/1036
Copyright: © 2015 Roehrig AT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective and Importance: Metastatic disease to the brain from breast cancer can be treated with multiple modalities. Determining effective therapies for select patients with brain metastases is critical to maximizing survival and quality of life. Stereotactic radiosurgery (SRS) can be an effective option for select patients with brain metastases, and is highly targeted with rapid fall-off of radiation dose outside the treatment zone. The following case illustrates the potential use of multiple Gamma Knife Radiosurgery (GKRS) sessions for a patient with metastatic breast cancer. In this specific case, repeat treatments led to prolonged survival and improved quality of life for a patient with multiple intracranial tumors from metastatic breast cancer.
Clinical Presentation: A 45-year-old woman presented to her primary care physician after an abnormal mammogram finding and worsening right hip pain. A breast biopsy and subsequent diagnostic imaging revealed metastatic breast cancer with metastases to her right femoral head, liver, and brain. She underwent GKRS for the three cerebellar lesions that were noted on MRI. Subsequently, she underwent additional GKRS treatments on 3 separate occasions for treatment of new lesions.
Intervention: The initial GKRS treatment targeted three lesions in the right cerebellum with 22 Gy to the 48% isodose line. Ultimately, she has been treated four times with GKRS for eight lesions and has received multiple systemic chemotherapy treatments for extra cranial disease. She is currently doing well at her 21-month follow-up, and is continuing navelbine.
Conclusion: A variety of treatment strategies are available and tailored treatment is necessary for each individual patient with brain metastases from breast cancer. This case report describes the time-course and favorable treatment outcome for a patient with recurrent breast cancer known brain metastases who underwent 4 separate GK treatment sessions along with systemic therapy.
Keywords
Stereotactic radiosurgery, Breast Cancer, Case Report, Survival, Prognostic Factors
Abbreviations
BCBM: Breast cancer brain metastases; GKRS: Gamma Knife™ radiosurgery; SRS: Stereotactic radiosurgery; WBRT: Whole brain radiation therapy; ER/PR+ : Estrogen receptor/Progesterone receptor positive; HER2: Human epidermal growth factor receptor 2; SBRT: Stereotactic body radiotherapy; RTOG: Radiation Therapy Oncology Group; RPA: Recursive Partitioning Analysis; KPS: Karnofski Performance Status
Introduction
One significant potential challenge in patients with metastatic breast cancer is the development of brain metastases. Brain metastases develop frequently, and outnumber the occurrence of primary intracranial tumors nearly 10:1 [1]. Nearly one third of all cancer patients will develop brain metastases during the natural history of their disease, totaling an annual occurrence in the US of approximately 170,000 new diagnoses of brain metastases. Brain metastases most commonly have origins of lung (50-60%), breast (15-20%), skin (5-10%) and gastrointestinal tract (4-6%) [2]. Breast cancer metastases to the brain occur in approximately 10-16% of patients diagnosed with primary breast cancer, and often presents 2-3 years after the initial diagnosis [3]. As new chemotherapy agents and other novel therapies lead to improvements in systemic disease control and improved overall survival, the frequency of the diagnosis of metastatic disease to the brain has been increasing [4].
Early studies frequently grouped all types of brain metastasis together when measuring survival outcomes [5-7]. Radiosurgery outcome studies are now further subdividing and investigating clinical outcomes based on tumor type and genetic mutations. Previous studies have revealed that specific breast cancer histology can influence the incidence, prognosis and median survival of patients [8]. Breast cancers with the genetic subtype triple negative (cancers without cell receptors for estrogen, progesterone, or HER-2) and the HER-2 subtype are associated with earlier development and more aggressive progression of metastatic disease to the brain [9]. Both of these cancers have a reported shorter median survival after brain metastasis: 4 months for triple negative subtype and 8.3 months for HER-2 subtype [10,11]. Other factors affecting prognosis are age, presence and location within the brain of multiple metastatic sites, tumor grade, and tumor metastasis size.
Treatment options for brain metastases are palliative in nature and include corticosteroids, surgical resection, chemotherapy, whole brain radiation therapy (WBRT), and stereotactic radiosurgery (SRS) [12]. For each clinical situation, a single modality or combination of modalities may be utilized. Depending upon the specific clinical scenario, WBRT or stereotactic radiosurgery (SRS) can be utilized as a monotherapy or in combination with one another. SRS is an effective treatment for brain metastases and in recent years has become more commonly utilized as a monotherapy and as the primary modality in many clinical situations. SRS allows for acceptable local tumor control when compared to surgical resection, and improved neurocognitive function when compared to whole brain radiotherapy [7,13]. In order to reduce the risk of late radiation toxicity and potential neurologic defects, SRS is increasingly used as a standalone treatment for patients with a small number or volume of metastatic growths [14].
In this article, we discuss the clinical course resulting in extended survival of a patient with brain metastases from breast cancer. This was achieved because of an initial small intracranial disease burden, and because of close follow up with imaging and four sequential Gamma Knife radiosurgery (GKRS) treatments as well as aggressive systemic chemotherapy treatment for her extra cranial disease. At the time of this report, she is alive with an excellent performance status and continues to be followed by the clinic 21 months after diagnosis of brain metastases.
Case Report
We present the case of a 45-year-old woman who initially presented to her primary care physician with complaints of joint pain, a recent history of "migraine headaches and dizziness", and an abnormal finding in her left breast on routine mammogram. A breast biopsy with subsequent lymph node dissection revealed ER/PR positive and HER-2 negative breast cancer and follow up staging studies including a PET-CT revealed multiple metastases to the liver and right femoral head. A brain MRI scan also revealed three cerebellar ring-enhancing lesions consistent with metastatic breast cancer. There was minimal mass effect, little vasogenic edema, and the largest of the tumors was 7 × 7 × 8 mm in size. She was referred to a radiation oncologist for a consultation and both whole brain radiotherapy and GKRS were discussed as possible treatment options. After discussing the risks and benefits of each treatment strategy, she decided to undergo GKRS and to initiate systemic chemotherapy. The patient was started on chemotherapy with doxorubicin and cyclophosphamide, followed bi-weekly with paclitaxel. She completed ten of the twelve planned treatments of paclitaxel before she developed an unacceptable sensori motor toxicity reaction and was forced to discontinue. She was also started on leuprorelin and dexamethasone shortly after her initial diagnosis. Approximately two weeks after diagnosis of the brain metastases, the patient underwent GKRS using a Leksell Gamma Knife Model C machine. On the day of treatment, her Karnofsky performance status (KPS) score was 80. A total of three lesions were treated with 22 Gy to the 48% isodose line (Figure 1). She tolerated the procedure well and her one-month follow-up MRI showed local control of all three tumors (no appreciable increase in tumor diameter on MRI scan), with a mild decreased size of the smallest (Figure 2). In addition to Gamma Knife (GK), the patient also received palliative external beam radiation to 3000 cGy in 10 fractions to her right femoral head and proximal femur, and subsequently underwent a total right hip arthroplasty due to a pathologic fracture.
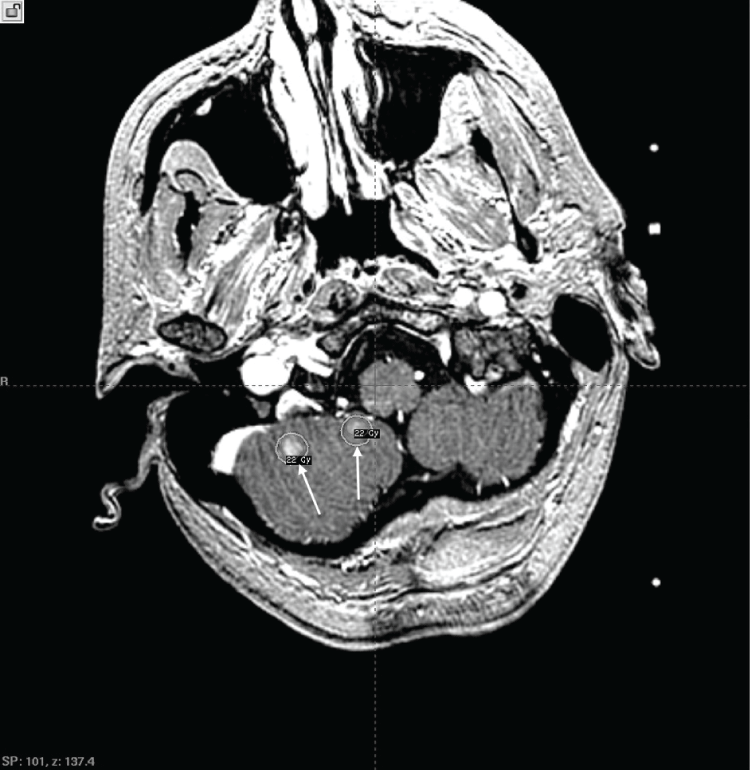
.
Figure 1: Axial T1 postgadolinium enhanced MRI. Arrows indicate two of three lesions in the right cerebellum and treatment plan isodose curves for the patient's initial GKRS treatment.
View Figure 1
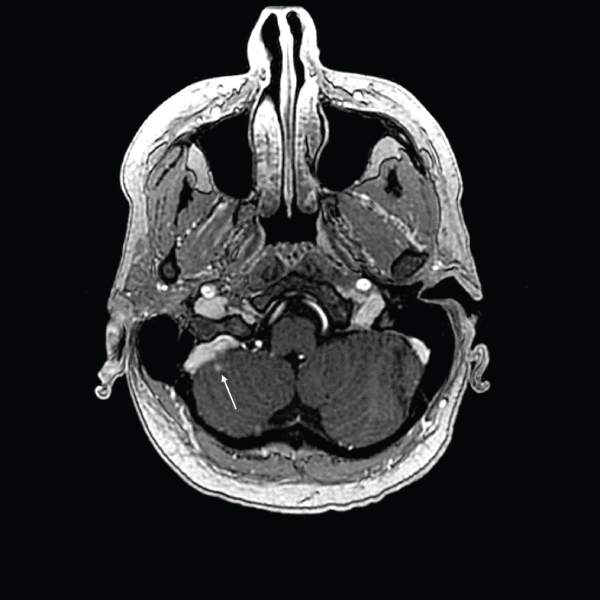
.
Figure 2: One-month follow-up MRI of patient's first treatment with GKRS showing recession of one lesion and stability of the other visible in Figure 1 (indicated by arrow).
View Figure 2
At four months post-treatment, the patient returned for routine follow-up and imaging. Her brain MRI was stable except for minor growth of a small occipital lesion (from 1mm to 2.5 mm diameter) that had been noted as suspicious 2 month prior but previously not treated. This new lesion was treated with GKRS at this time (2nd GKRS treatment), which was tolerated well, and did not have evidence of recurrent disease in her brain for another 10 months on surveillance MRI imaging. MRI scans were obtained at 8-12 week intervals over this time frame for surveillance.
Fourteen months after her initial diagnosis, the patient experienced a return of neurologic symptoms. She described headaches occurring 4-5 times per week, and a sense of mental "fogginess" that she recalled feeling previously before her first GK treatment. Her MRI showed growth of an additional three new metastases, ranging from 2mm to 1cm in diameter. She again underwent GKRS for these lesions (3rd GKRS treatment). At the same time, her systemic disease had progressed with multiple new metastatic lesions in her liver, an extensive recurrence that was thought to be seeding her newly-developed brain lesions.
Follow-up was stable for 3 months when an MRI showed development of two new lesions, each about 1-3 mm in diameter (Figure 3). Gamma Knife was repeated for a fourth time (4th GKRS treatment), with subsequent excellent local control on follow up imaging with brain MRI at 2-3 month intervals (Figure 4). Following GK, her subsequent MRI scans have been stable until present day, 21 months after her initial diagnosis.
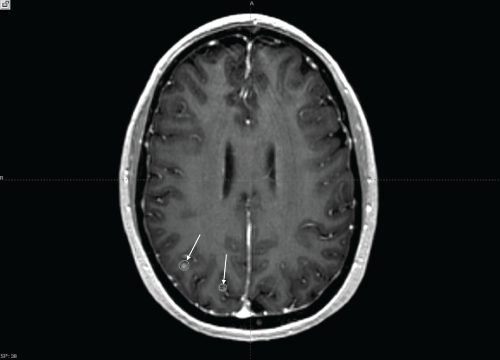
.
Figure 3: Axial T1 post gadolinium enhanced MRI. Arrows indicate two additional lesions in the right parietal lobe and treatment plan isodose curves for the patient's most recent GKRS treatment.
View Figure 3
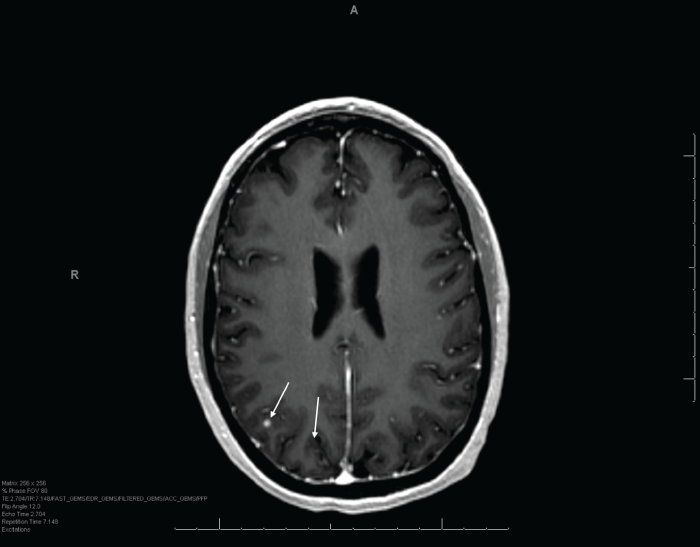
.
Figure 4: One-month follow-up MRI of patient's most recent GKRS treatment showing stability and recession of two parietal lesions (indicated by arrows).
View Figure 4
At the time of her last known follow-up at 21 months after her initial diagnosis of cancer and diagnosis of brain metastases, her systemic disease had recurred. While recent PET/CT scans showed no signs of further bone recurrences, her liver disease had produced evidence of new metastases. She continues to be a good candidate for chemotherapy and has been started on vinorelbine.
Despite her aggressive past therapies, she continues to live independently and is largely pain-free. This case demonstrates that an acceptable quality of life can be maintained for select patients with multifocal and extensive and recurrent brain metastases from breast cancer by offering multiple sequential SRS treatments.
Discussion
Brain metastasis clinical prognostic factors affecting treatment
In 1989, several key prognostic factors for patients with metastatic cancer to the brain were identified by the Radiation Therapy Oncology Group (RTOG) and associated with increased survival. These four factors include Karnofsky Performance Status (KPS), presence of active cancer systemically (outside of the brain), age, and extent of metastatic disease [5]. The authors of the study reported that patients who had a KPS score of 70-100, absence of active systemic disease, age < 60 years, and metastases limited to the brain had a 52% predicted probability of surviving 200 days. Furthermore, patients with three of the four positive factors had a 33% chance of 200-day survival. These factors and their prognostic ability were confirmed in 1997, with publishing of a recursive partitioning analysis (RPA) including classes I, II and III to group patients by prognosis [15]. These classes can have statistically significant differences in overall survival rates from one another and the reported clinical factors have been shown in multiple studies to be prognostic. As an example, in 2007, Niwinska et al. looked at survival in 31 breast cancer patients with 1-2 brain metastases and found that RPA class was the most significant prognostic indicator of survival [16]. Furthermore, they found that survival differed significantly between Classes I, II and III (28 months, 13 months, and 3 months, respectively; P = 0.0001).
The prognostic factors identified by the RTOG have been shown to be helpful in treatment decision making. The patients with the best overall survival are those who exhibit the factors previously described, as well as those who have a limited number (and limited tumor volume) of brain metastases. When an SRS boost was added to WBRT, a study by Gasper et al. found a survival benefit in patients with an RPA Class I [15]. This study also found a survival benefit in patients with a single brain metastasis. Retrospective studies have not found a significant survival benefit to adding an SRS boost to WBRT for patients not in RPA Class I, but recent studies indicate local control has been found to be improved with SRS [5,17].
Histology and breast cancer occurrence and survival
Breast cancer remains among the top three cancers with the highest incidence of metastasis to the brain, along with non-small cell lung cancer (NSCLC) and melanoma. About 40% of patients with NSCLC and nearly 50% of those with metastatic melanoma (< 2% of all melanoma cases) will develop brain metastases, while approximately 30% of patients with breast cancer will develop brain metastases [7,18,19]. Prognosis remains poor for all patients diagnosed with brain metastases, with average survival rates reported under 12 months. Survival rates have been found to correlate well with the previously discussed RTOG prognostic factors, but can vary somewhat for cancers of different tissues of origin. NSCLC, for example, generally has an average survival of 7 months, while melanoma can have a median survival ranging from 4-7 months [16,17]. Untreated, breast cancer brain metastases has a median survival of only 1 month, but current treatment regimens can extend that to 2.5 months with steroid treatment alone and up to 12 months with surgical resection and radiotherapy [20].
Histology and breast cancer subtype play a role in overall occurrence and tendency to metastasize to the brain. Although ER-positive and HER2-negative subtypes may have an occurrence of metastases in 5-10% of cases, other subtypes have a significantly increased likelihood of developing brain metastases [21]. For example, triple negative and HER2-positive subtypes, although representing a minority of breast cancers, can have brain metastasis occurrence as high as 20% and 25-50%, respectively [22]. This remarkable difference between subtypes has been hypothesized to be linked to better control of systemic disease, particularly for HER2-positive subtypes by use of the targeted therapy trastuzumab [23]. Overall, the incidence of clinically determined BCBM may be much smaller than the true incidence, including brain metastases with no appreciated neurologic symptoms. A retrospective study by Niwinska et al. looked at a group of 222 breast cancer patients with brain metastases divided into three biological subgroups: HER-2 Positive, luminal, and triple negative [24]. They assessed patient survival based on the biologic subtype, recursive partitioning analysis class and the use of systemic treatment after WBRT. The results of the study showed that RPA class had the most significant prognostic effect on survival (as mentioned above), but that median survival differed between the biological subtypes as well. The authors found that triple negative subtype had the shortest median survival at 3.7 months, followed by HER-2 positive and luminal subtypes, at 9 and 15 months, respectively. From their results, the authors were able to conclude that HER-2 positive and triple negative subtypes have an increased occurrence of brain metastases and that performance status was a key prognostic indicator of survival.
Side effects of SRS
As with any brain directed therapy, SRS has benefits and side effects associated with its use. It allows for minimally invasive treatment of small/deep lesions, it is an outpatient procedure, it allows for treatment of multiple lesions during the same session, the recovery time is usually less than 1-2 days, and there is the potential avoidance of the neurocognitive side effects from whole-brain radiation [7]. The main risk of radiosurgery is a small risk of radiation injury to surrounding brain tissue and treatment related changes such as tissue edema (which in some cases can lead to a treatment related change in the enhancing region on brain MRI that appears similar to progression of disease) [25,26]. Pseudo-progression has also been reported in patients treated for recurrent glioblastoma multiforme with SRS and temozolomide, with 13-32% of patients exhibiting some degree of pseudoprogression [27]. Minor adverse effects of SRS range from common complications such as screw-site soreness, tissue swelling, and headache from head-frame placement [2]. There are also side effects associated with the use of corticosteroids, such as dependency, psychosis, diabetes, insomnia, weight gain and immunosuppression. Many of these side effects of treatment are present in WBRT as well as SRS, although unlike WBRT, SRS has not been associated with as high of risk of treatment related neurocognitive decline [28].
SRS and other treatment options
The role of SRS in conjunction with other treatment modalities (such as whole brain radiotherapy) for treating one or several brain metastases remains a subject of ongoing research study and clinical decisions should be tailored to each unique clinical situation. There is no clear evidence that surgery plus WBRT improves survival over treatment with SRS with WBRT, according to a randomized, controlled trial by Kocher et al [21]. In addition, clinicians are wary of the reported potential side effects of WBRT, such as long-term neurocognitive decline [12,29]. Surgical resection can plan an important role in some clinical situation (where large tumors are causing shifting of intracranial structures or a pathologic diagnosis is needed), but surgery is not always a possible on tumors that are within or in close proximity to critical structures such as those that control speech, language, sensation or motion. Welzel et al. observed neurocognitive decline in patients who received WBRT for brain metastases, and preferred the option of salvage treatments with SRS for recurrences until WBRT was deemed necessary [30]. Advocates in favor of WBRT in combination with SRS argue that the neurocognitive defects caused by the higher recurrence rate of brain metastases associated with SRS treatment alone can in some situations be more severe than those caused by WBRT. A recent randomized trial by Brown et al. has disputed this fact, stating that initial treatment with SRS and close follow-up (with retreatment with SRS or WBRT as needed) is preferred to initial WBRT in patients with one to three metastases [28].
Conclusion
This report illustrates the potential for an extended overall survival and acceptable quality of life for select patients with brain metastases from breast cancer who are treated with multiple sequential GKRS sessions. For this select case, treatment was accomplished with close follow up at 2-3 month intervals including brain MRI, and repeat SRS was offered when new brain metastases were diagnosed (a total of 4 GKRS sessions were completed). In addition, she underwent a sequence of systemic therapies for her extra cranial metastatic disease. GKRS treatments produced symptomatic relief with good duration of local control and were shown to be well tolerated with each sequential treatment. She continues to be followed clinically and is able to live independently with an appreciable quality of life.
Acknowledgement
We would like to acknowledge Eric Reynolds and Jill Adams for their help and support, as well as the entire Gamma Knife of Spokane and Cancer Care Northwest research staff, for their contributions to this manuscript.
Ethical Statement
This case report was conducted with the informed, written consent of the patient. There are no conflicts of interest to declare.
References
-
Arnold SM, Patchell RA (2001) Diagnosis and management of brain metastases. Hematol Oncol Clin North Am 15: 1085-1107, vii.
-
Newton HB (2002) Chemotherapy for the treatment of metastatic brain tumors. Expert Rev Anticancer Ther 2: 495-506.
-
Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167: 913-920.
-
Santarelli JG, Sarkissian V, Hou LC, Veeravagu A, Tse V (2007) Molecular events of brain metastasis. Neurosurg Focus 22: E1.
-
Diener-West M, Dobbins TW, Phillips TL, Nelson DF (1989) Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys 16: 669-673.
-
Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, et al. (1996) A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 78: 1470-1476.
-
Auchter RM, Lamond JP, Alexander E, Buatti JM, Chappell R, et al. (1996) A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys 35: 27-35.
-
Arslan UY, Oksuzoglu B, Aksoy S, Harputluoglu H, Turker I, et al. (2011) Breast cancer subtypes and outcomes of central nervous system metastases. Breast 20: 562-567.
-
Berghoff A, Bago-Horvath Z, De Vries C, Dubsky P, Pluschnig U, et al. (2012) Brain metastases free survival differs between breast cancer subtypes. Br J Cancer 106: 440-446.
-
Eichler AF, Kuter I, Ryan P, Schapira L, Younger J, et al. (2008) Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer 112: 2359-2367.
-
Hayashi N, Niikura N, Masuda N, Takashima S, Nakamura R, et al. (2015) Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat 149: 277-284.
-
Suh JH (2010) Stereotactic radiosurgery for the management of brain metastases. N Engl J Med 362: 1119-1127.
-
Lee SR, Oh JY, Kim SH (2015) Gamma Knife radiosurgery for cystic brain metastases. Br J Neurosurg 1-6.
-
Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, et al. (2000) Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys 47: 993-999.
-
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, et al. (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37: 745-751.
-
Niwinska A (2007) Surgery and radiotherapy of brain metastases in breast cancer patients-an analysis of survival and prognostic factors. Nowotwory Journal of Oncology 57: 50e-54e.
-
Hazard LJ, Jensen RL, Shrieve DC (2005) Role of stereotactic radiosurgery in the treatment of brain metastases. Am J Clin Oncol 28: 403-410.
-
Lin CH, Hsu KH, Chang SN, Tsou HK, Sheehan J, et al. (2015) Increased survival with the combination of stereotactic radiosurgery and gefitinib for non-small cell lung cancer brain metastasis patients: a nationwide study in Taiwan. Radiat Oncol 10: 127.
-
Wattson DA, Sullivan RJ, Niemierko A, Merritt RM, Lawrence DP, et al. (2015) Survival patterns following brain metastases for patients with melanoma in the MAP-kinase inhibitor era. J Neurooncol 123: 75-84.
-
Soffietti R, Ruda R, Trevisan E (2008) Brain metastases: current management and new developments. Curr Opin Oncol 20: 676-684.
-
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, et al. (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 29: 134-141.
-
Aversa C, Rossi V, Geuna E, Martinello R, Milani A, et al. (2014) Metastatic breast cancer subtypes and central nervous system metastases. Breast 23: 623-628.
-
Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, et al. (2011) Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17: 4834-4843.
-
Niwinska A, Murawska M, Pogoda K (2010) Breast cancer brain metastases: differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 21: 942-948.
-
Babu R, Huang PP, Epstein F, Budzilovich GN (1993) Late radiation necrosis of the brain: case report. J Neurooncol 17: 37-42.
-
Ruzevick J, Kleinberg L, Rigamonti D (2014) Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev 37: 193-201.
-
Gunjur A, Lau E, Taouk Y, Ryan G (2011) Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: a retrospective analysis. J Med Imaging Radiat Oncol 55: 603-610.
-
Brown PD, Asher AL, Ballman KV, Farace E, Cerhan JH, et al. (2015) A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol 33.
-
Li J, Bentzen SM, Renschler M, Mehta MP (2007) Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol 25: 1260-1266.
-
Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, et al. (2008) Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys 72: 1311-1318.





