Tuberculin skin testing (TST) response as a predictive tool for development of pulmonary tuberculosis (PT) in Human Immunodeficiency Virus type-1 (HIV-1) infected subjects, is likely to be more valuable at early stage of illness in order to adapt timely management strategy. Earlier reports on HIV-1 infected blood donors with history of oral iron intake and biochemical evidence of iron overload documented development of high incidence of PT on follow up.
A group of HIV-1 infected asymptomatic blood donors, belonging to replacement or voluntary categories, were subjected to TST and anergy testing using a commercial panel of recall antigens along with estimation of serum iron parameters, peripheral CD4+ T cell count, plasma viral load and assessment of in vitro production of interferon gamma (IFN-γ) by peripheral blood mononuclear cells (PBMC) in response to PHA, BCG and PPD.
The replacement category of donors showed biochemical evidence of iron overload with high proportion (60%) demonstrating negative TST response on initial testing at enrollment but positive response on repeat testing after 2-3 weeks (boosting) or after 1 year (conversion) consequent to normalization of iron parameters. However, such initial negative TST response at enrollment in donors showing boosting or conversion was not associated with evidence of anergy to recall antigens but corroborated with impaired IFN-γ production by PBMC to PPD challenge that could be reversed on addition of exogeneous recombinant interleukin 12 (rIL-12). Majority (63.6%) of replacement donors showing boosting reaction or conversion developed PT on follow up.
Subclinical iron overload may mask TST response due to impaired production of IFN-γ by PBMC to M. tuberculosis specific antigens that could be related to inadequate cooperation of IL-12 from macrophages. Such masking of TST associated with iron overload may hamper predictive value of TST for future development of PT.
TST, Anergy, Boosting, Gamma interferon, Pulmonary Tuberculosis, HIV-1
Co-infection of Human Immunodeficiency Virus (HIV) and Mycobacterium tuberculosis (M.tb) is a major public health problem in developing countries with high prevalence of tuberculosis (TB) [1]. It has been advocated that most of the deaths resulting from TB in HIV infected patients are preventable by timely diagnosis and treatment at the latent stage of infection (Latent TB infection or LTBI) [2]. Despite a compromise with specificity, mainly due to confounding effect of BCG vaccination, the age old tuberculin skin test (TST) employing protein purified derivative (PPD) of M.tb has been the most extensively used tool worldwide for the diagnosis of TB [3]. Furthermore, it has been advocated that assessment of cutaneous delayed type hypersensitivity (DTH) response to multiple recall antigens (anergy testing) could be a useful adjunct to TST for assessing general immune competency in HIV infected individuals [4]. Both these in vivo tests are based on production of the cytokine interferon gamma (IFN-γ) by peripheral CD4+ T lymphocytes with cooperative signal of interleukin 12 (IL-12) from macrophages [5]. Earlier studies on HIV type 1 (HIV-1) infected blood donors from northern India during the pre-ban era on remunerated blood donation practice in the country revealed biochemical evidence of subclinical iron overload in many of them due to excess oral iron intake as a precautionary measure to maintain hemoglobin level and were characterized by development of high incidence of pulmonary tuberculosis (PT) on follow up [6]. The present report attempts an evaluation of the predictive value of TST towards development of PT on follow up in an asymptomatic group of ART naïve HIV-1 infected blood donors in relation to baseline status of serum iron parameters, anergy testing response and production of IFN-γ by peripheral blood mononuclear cells (PBMCs) in response to mitogen phytohemagglutinin (PHA), live attenuated vaccine strain of Bacillus Calmette Guerrin (BCG) bacilli and M.tb specific antigen viz. protein purified derivative (PPD) in presence or absence of exogenous IL-12.
The present study was prospective in nature, carried out on a group of HIV-1 seropositive blood donors (referred as HIV-1 positive donors subsequently) enrolled over a five year period following implementation of legal ban on remunerated blood donation. Each of the study subjects was followed up clinically for a minimum period of 10 years to detect development of PT during the follow up period as the outcome of interest. Prior approval of the protocol of the study was obtained from independent institutional review board including ethical clearance.
Between December 1998 and November 2003, various blood banks in the National Capital Territory of Delhi and northern India were requested to refer the blood donors detected to be seropositive for HIV-1 infection at their centers to AIDS Reference Laboratory, National Center for Disease Control, Delhi. The referred donors, all males between 20-35 years, were provided patient information sheet and informed consent was obtained from them for the study.
Information was obtained from the referring blood banks to find out the category of the HIV-1 positive donors assigned by them as per national guideline i.e. (i) 'voluntary' donor, defined as an unpaid blood donor donating blood voluntarily in a blood bank or in a blood donation camp or (ii)'family/replacement' donor (commonly referred as replacement donor) defined as a member of the family or friend of a patient, who had donated blood in replacement of blood needed for the particular patient without involvement of any monetary or other benefits from any source [7]. A confidential interview was conducted for each HIV-1 positive donors to collect information on HIV-1 related risk behaviors viz. unprotected sex with heterosexual and/or homosexual partner, parenteral drug abuse or receipt of blood transfusion. Considering that unprotected sexual exposure to female commercial sex workers (CSWs) was the only HIV-1 related risk behavior disclosed by some of the donors, they were further enquired about the time of such exposures although precise time of acquisition of HIV-1 infection could not be ascertained from the HIV-1 positive donors with history of recurrent high risk exposures. History regarding regular intake of iron tablet was obtained from the donors although information regarding quantity and duration of iron tablet intake could not recorded due to lack of definite recall. In addition, history regarding consumption of alcohol was elicited from the donors although no consideration was given in terms of quantity or brand. Evidence of BCG vaccination was elicited from the donors based on inspection of visible scars [8]. Additional information was collected from the HIV-1 positive donors regarding documented history of treated or untreated/partially treated tuberculosis, occupation in any health care organization, residence in shelter homes and sharing of residence with a treated or untreated person with diagnosis of TB.
Initially, about 3 ml of blood was collected from each of the referred donors, 1 ml in plain sterile tube to yield serum for confirmatory serological testing of HIV-1 infection by Western blot and 2 ml in EDTA vial for peripheral CD4+ T lymphocyte count (referred subsequently as CD4 count in the paper) using labeled monoclonal CD4/CD3 reagent and analysis by FACS count machine (BD Biosciences, San Jose, CA, USA).
All the donors confirmed to be seropositive for HIV-1 infection were referred to clinical specialists at designated referral centers for clinical staging as per WHO guidelines being followed at that time in the country based on clinical, laboratory and radiological evidence of any AIDS related illness (ARI) including pulmonary or extra-pulmonary tuberculosis [9].
The HIV-1 positive donors were subjected to screening for Syphilis by Treponema pallidum hemagglutination (TPHA) test (Fujirebio, Tokyo, Japan) and for Hepatitis B virus (HBV) infection employing commercial Enzyme Linked Immunosorbent Assay (ELISA) for Hepatitis B surface antigen (HBsAg), antibody to Hepatitis B surface antigen (anti-HBs) and antibody to Hepatitis B core antigen (anti-HBc) while screening for HCV infection was carried out employing the 3rd generation micro-ELISA kits, all from commercial sources (Abott laboratories, North Chicago, Illinois, USA) as described earlier [6].
Only the HIV-1 positive donors with negative serologic evidence of HBV, HCV and Syphilis co-infections, CD4+ T cell counts > 550 cells/cumm i.e. 10% above the cut off value of 500 cells/cumm as the lower limit of unimpaired CD4 cell count [10] and diagnosed by the clinicians to be at asymptomatic stage for HIV-1 infection based on clinical, radiological and relevant laboratory investigations were enrolled for purpose of the present study.
Estimation of additional laboratory parameters: Estimation of albumin as well as levels of iron, ferritin and transferrin saturation (TS) percentage in serum were carried out by techniques as described earlier [6]. Plasma viral load assay in stored blood was estimated using commercial kit with sensitivity level of 400 copies/ml (Roche Amplicor, Branchbury, NJ, USA) [6].
In vitro IFN-γ production by peripheral blood mononuclear cells (PBMCs): This was carried out one day prior to TST and anergy testing at enrollment employing whole blood assay technique [11]. Briefly, one ml of venous blood, collected in sterile tube containing phenol free heparin (10 U/ml) was diluted 1 in 10 with sterile RPMI 1640 tissue culture medium containing 10 mM HEPES buffer, 2 mM L-glutamine, 100 IU of penicillin/per ml, 100 μg of streptomycin/ml, 0.25 gm amphotericin B/ml and 5% (vol/vol) heat inactivated fetal calf serum (FCS). All reagents were checked for endotoxin contamination by the Limulus amebocyte lysate assay. Diluted blood was dispensed in 96 well flat bottom tissue culture plate in six wells (180 μl) per sample. Five of the six wells were charged with 20 μl volumes of mitogens or antigens in the following concentrations (i) Phytohemagglutinin (PHA) (Sigma chemicals, USA) 10 μg/ml, (ii) Human PPD (Statens Serum Institute, Copenhagen, Denmark) 10 μg/ml (iii) Human PPD, 10 μg/ml plus 10 ng/ml of recombinant human interleukin-12 (rIL-12, R&D Diagnostics, Minneapolis USA) (iv) BCG vaccine (BCG Vaccine Laboratory, Chennai, India), 15 μg/ml and (v) BCG vaccine, 15 μg/ml plus 10 ng/ml of rIL-12, while the sixth well received 20 μl of RPMI only to check spontaneous production of IFN-γ. The plate was incubated at 37 ℃ in humidified 5% CO2 atmosphere. The supernatant was harvested after 3 days of incubation from the first well i.e. the well with PHA stimulation while for the remaining wells containing PPD and BCG, treated or untreated with rIL-12, the incubation was extended up to 5 days before the supernatants were harvested. The harvested supernatants from all the wells were stored at -70 ℃ for estimation of IFN-γ by commercial ELISA kit with sensitivity of 8 pg/ml and detection range of 15.6-1000 pg/ml (R&D Diagnostics, Minneapolis USA). Samples showing OD values above detectable range were diluted 1 in 10 and were re-quantified.
Tuberculin skin testing (TST) and anergy testing: TST was performed by intradermal injection of 0.1 ml of 5 TU PPD (Statens Serum Institute, Copenhagen Denmark) introduced on the volar aspect of the arm using Mantoux method. An induration of at least 5 mm was considered positive TST response (baseline TST positives) for the HIV-1 positive donors [12]. Simultaneously, the individual was subjected to anergy testing by multiple skin puncture technique on the volar aspect of the other arm to a panel of recall antigens using a commercial multi-test CMI test kit (Connaught Merieux, Lyon, France) as described earlier [13]. The multi-test CMI kit contained a multiple applicator to deliver seven test antigens and glycerol as negative control by percutaneous puncture device. The antigens included (1) Tetanus toxoid (equivalent to 550,000 Merieux Tetanus units/ml) (2) Diphtheria toxoid (equivalent to 1,100,000 Merieux units/ml) (3) Streptococcus group C antigen (equivalent to 2000 Merieux units/ml), (4) Candida albicans antigen (equivalent to 2,000 Merieux units/ml) (5) Trychophyton (150 Merieux units/ml) (6) Proteus mirabilis (equivalent to 150 Merieux units/ml) (7) Old tuberculin (equivalent to 300,000 US Tuberculin units) and (8) glycerol 70% w/v as negative control. Readings of anergy testing was taken in two perpendicular directions and mean of the two readings was recorded. Mean induration of 2 mm or more to any test antigen was considered as positive reaction as per manufacturers guidelines. Interpretation was made in terms of number of test antigens to which positive reactivity was observed and a DTH score of an individual was calculated as the sum of the means of diameters of all the positive reactions. Anergy was defined as lack of positive response (mean DTH score < 2 mm) to any of the seven antigens included in the panel [14]. Both TST and anergy test reactions were read at the same point of time by the same trained personnel. Initially readings were taken by both vernier caliper and transparent ruler that showed > 98% agreement between the two methods up to a precision of 1 mm and therefore the later method was employed for subsequent recording of the readings. However, in order to avoid subjective error, readings between 0 to < 2 mm were grouped together as < 2 mm.
Repeat TST and anergy testing: Repeat TST was carried out according to the protocol of two-step TST based on the guidelines of CDC [15]. In all cases with negative TST result in first-step, TST was repeated after an interval between 2-3 weeks (second-step TST). Any recording of positive reaction in second step TST in a case with negative result in the first step TST was considered as 'boosting' phenomenon and such cases were also included as baseline TST positives while donors negative for TST by both the tests in two-step TST were considered as baseline TST negatives. All the donors available for follow up (excluding the drop outs) were subjected to additional TST after one year. A positive TST result on additional testing after one year in a baseline TST negative donor was considered as 'conversion' while any negative TST result after one year in baseline TST positive donors was defined as 'reversion'[16]. Anergy testing was carried out along with TST as per the similar two-step protocol in all subjects to record any change in response to individual recall antigens. However, due to logistic constraints, anergy testing could not be repeated after one year as carried out in case of TST to record any change in the response.
Follow up laboratory studies: All the laboratory parameters viz. serum levels of albumin, iron, transferrin saturation and ferritin were repeated at the time of second step TST. The CD4 counts and plasma viral load were repeated for all the donors available at follow up after an interval of one year following enrollment at the time of recording TST conversion.
Follow up clinical studies: All the HIV-1 positive blood donors enrolled with the identified clinicians in referral hospitals were requested to report to the clinicians for development of symptom on follow up, regardless whether they were AIDS related or not. Development of pulmonary tuberculosis (PT), diagnosed by the clinician based on clinical findings, chest X-ray, microscopy and culture of sputum [17] was considered as the end point in the present study for calculation of symptom free (PT free) duration. In order to avoid confounding effect of antiretroviral therapy (ART) introduced for other categories of ARI developing before PT, very few cases developing PT after development of non-PT category of ARI were excluded in the study and only those cases developing PT as the first presenting ARI were considered.
A total of 100 HIV negative age matched male voluntary blood donors, randomly selected from the blood banks referring the HIV-1 positive donors and screened negative for HBV, HCV and Syphilis infections were selected as controls. These control donors were subjected to clinical assessment and relevant laboratory investigations at enrollment and at follow up as in case of HIV-1 positive donors. TST was carried out using the same protocol as in case of HIV-1 positive donors except that for the HIV negative controls an induration of ≥ 10 mm was considered as positive TST as per standard guideline [12]. However anergy testing was carried out at enrollment only for the HIV negative controls.
Statistical analysis was carried out employing SPSS package version 20. Characteristics of the two categories of blood donors were compared using χ2 test for categorical variables and student's t-test or one way ANOVA followed by post-hoc test with Bonferroni adjustment for continuous variables with normal distribution characteristics. Continuous variables not following normal distribution were compared by Kruskal Wallis test followed by multiple comparisons using Mann Whitney test with Bonferroni adjustment. P value < 0.05 was considered as statistically significant and it was adjusted for multiple comparisons. Fisher's exact test was used if any of the expected cell frequency was less than five. Agreement between TST and OT response was expressed as k value, where values of > 0.75, 0.4-0.75 and < 0.4 were considered as excellent, fair and poor agreements respectively [18]. Correlations between continuous variables were calculated by Pearson's correlation coefficient. Rate of fall of CD4+ T cell count (per month) and increase in viral load (per year) was calculated based on values obtained at enrollment (at the point of first TST) and at the time of repeat TST performed at one year of post-enrollment period.
During the period between 1998 and 2003, a total of 144 HIV-1 donors comprising of 70 donors belonging to replacement category and 74 donors belonging to voluntary category were enrolled for the study as per inclusion criteria. Theses donors were all males between 22 and 35 years of age (mean age 26 ± 2.4 and 22 ± 3.6 respectively, P = NS). Positive history of regular intake of oral iron tablet was disclosed by many (42 out of 70, 60%) of the replacement category of HIV-1 positive donors. The rational for intake of oral iron, as disclosed by the donors, was a precautionary measure to maintain hemoglobin level although they did not have any documented prior clinical or laboratory indication of iron deficiency. However, only one of the HIV-1 positive donors in 'voluntary' category and none of the HIV negative donors gave history of such iron intake. Frequency of alcohol consumption was higher (48 out of 70, 68.6%) in the replacement category of donors compared to the voluntary donors (28 out of 70, 40%; p < 0.05).
Only 32 out of 144 (22.2%) HIV-1 positive donors, comprising of 12 out of 70 (17.1%) in replacement category and 20 out of 74 (27%) in voluntary category disclosed history of unprotected sexual exposure to female CSW between 2-8 months prior to HIV-1 positive blood donation while 2 donors belonging to the replacement category disclosing multiple such exposures during preceding 2 years. None of the HIV-1 positive donors had any record of HIV test result available with them prior to donation of blood. Visible scar as evidence of BCG vaccination was recorded to be equally high in both the categories of donors (> 90% in both categories).
TST and anergy testing: Baseline two-step TST showed positive results in 10 out of 70 (14.3%) and 12 out of 74 (16.2%) donors in replacement and voluntary categories respectively in first-step TST (P = NS). However, additional 36 out of 60 (60%) donors in replacement category and 4 out of 62 (6.5%) donors in voluntary category with negative results in first-step TST demonstrated positive response in second-step TST (boosting phenomenon) (Odd's ratio 21.8, 95% CI 6.9-67.8; P < 0.001). Eight out of 22 (36.3%) donors in replacement category (excluding 2 drop outs) and 2 out of 55 (3.6%) of voluntary donors (excluding 3 drop outs) with negative reactivity at both the steps of two-step TSTs at enrolment showed conversion i.e. positive TST response at one year of post-enrollment follow up (χ2 = 15; p < 0.001) (Figure1). None of the donors with positive TST result at baseline, whether at first-step TST or at second-step TST, showed reversion to negative TST result on subsequent follow up TST at one year of post-enrollment.
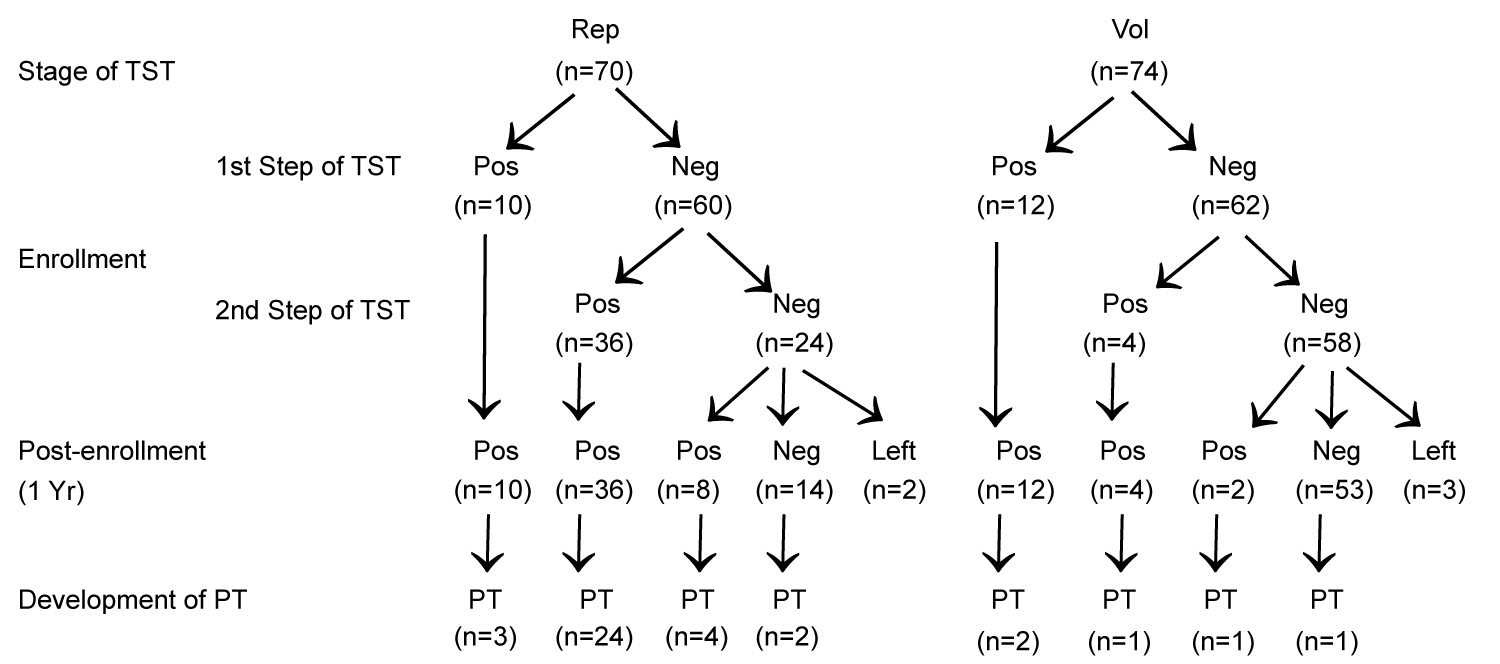 Figure 1: Flow chart of the TST reactivity pattern in replacement (Rep) and voluntary (Vol) categories of blood donors and development of pulmonary tuberculosis (PT) on follow up.
View Figure 1
Figure 1: Flow chart of the TST reactivity pattern in replacement (Rep) and voluntary (Vol) categories of blood donors and development of pulmonary tuberculosis (PT) on follow up.
View Figure 1
Comparison of TST response to various antigens included in anergy testing panel by Multi-test CMI kit in the HIV-1 positive donors showed high degree of quantitative agreement between various patterns of TST results and parallel response to OT antigen in anergy testing. However, there was divergence in the pattern of response between TST and anergy testing for other remaining six antigens included in the anergy test panel in donors showing boosting or conversion. As opposed to negative TST results demonstrated by donors at first step TST, the donors showing 'boosting' or 'conversion' on follow up testing showed positive response to one or more of the remaining 6 antigens of anergy test panels at the same point of testing (Table 1).
Table 1: Correlation between various patterns of TST response and anergy testing results to recall antigens. View Table 1
Out of 100 HIV negative controls, a total of 16 (16%) showed positive TST response at first TST of enrollment with 2 out of the remaining 84 (2.4%) first-step TST negative donors showing boosting reaction at second-step TST. Conversion was recorded in 2 of the remaining 80 (2.5%) donors available on follow up (excluding 4 drop outs). The response to OT antigen in anergy test panel was comparable to that of TST results similar to that observed in HIV-1 positive donors. However, as opposed to HIV-1 positive donors, anergy test responses in the 4 donors showing 'boosting' or 'conversion' were concordant to that of TST result in HIV-1 negative donors i.e. complete anergy at the point of first-step TST but positive reaction for three or more antigens at the point of boosting' or 'conversion' (data not shown in the table).
Iron parameters at enrollment and their correlation with degree of positive TST response: Assessment of serum iron parameters at enrollment showed elevated levels in the replacement category of donors at the time of first step TST that came down to normal levels when assessed at the time of second step TST while the voluntary donors showed unaltered levels comparable to controls at both steps of enrollment TST (Table 2). There was no correlation between serum ferritin level and degree of TST response (mean diameter) in cases showing positive response in first-step TST (n = 10) in replacement category of donors. However, there was a positive correlation between serum ferritin level and degree of increase (difference of diameters) in responses between initial negative TST and subsequent positive TST response in the 44 cases showing booster (n = 36) or conversion (n = 8) phenomenon (Figure 2).
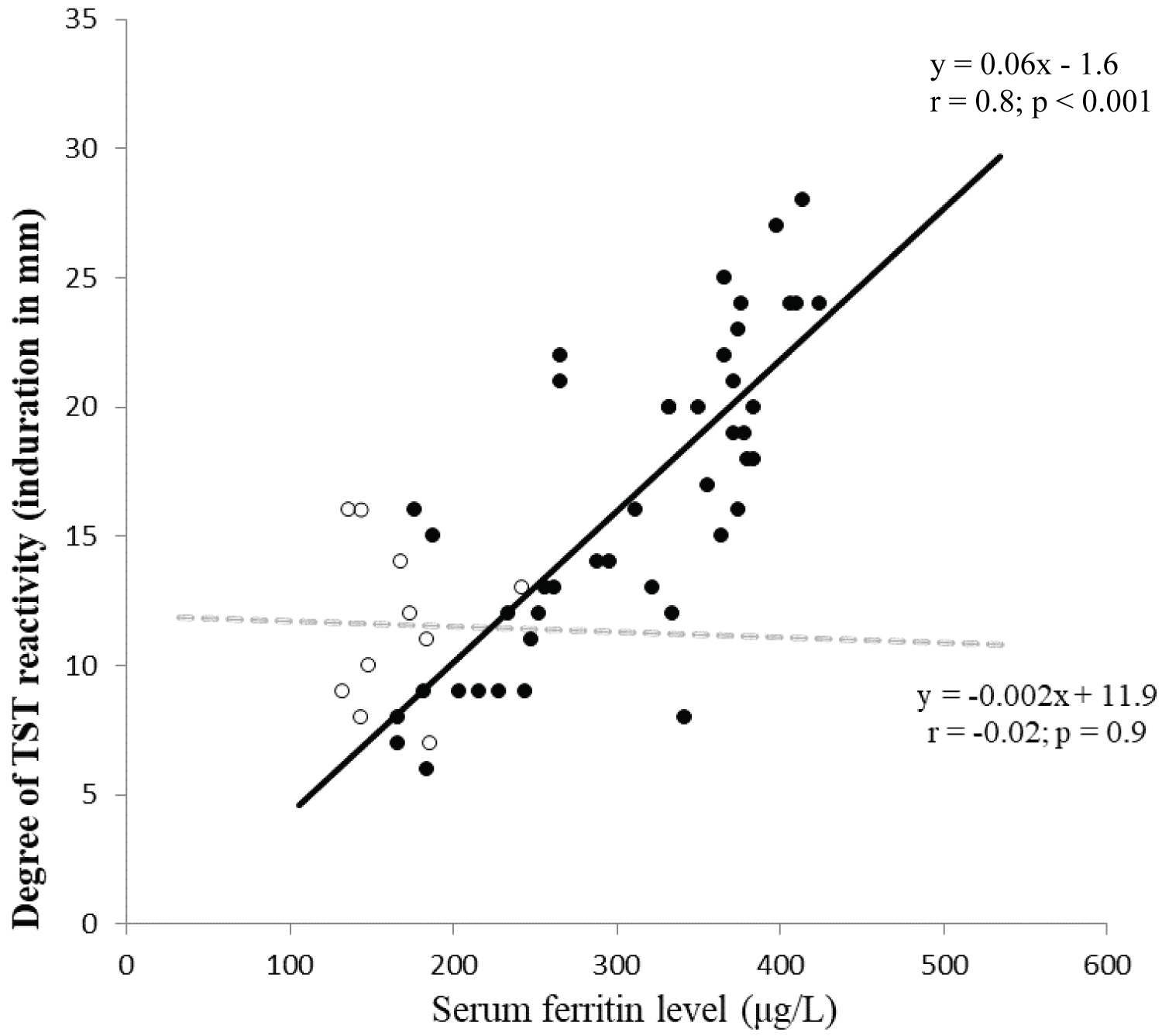 Figure 2. Correlation between serum ferritin level (μg/L) and degree of TST reactivity (induration diameter in millimetre).
Figure 2. Correlation between serum ferritin level (μg/L) and degree of TST reactivity (induration diameter in millimetre).
• Indicates difference in degree of TST reactivity between 1st (negative) and 2nd (positive) TST in cases showing 'boosting' or 'conversion'
○ Indicates degree of TST in cases showing positive reactivity in 1st TST
View Figure 2
Table 2: Assessment of biochemical, immunological and virological parameters in HIV-1 positive donors at enrollment (values expressed as mean ± SD). View Table 2
CD4 count and viral load at enrollment: There was no difference in absolute CD4 cell count and plasma viral load between the two categories of HIV-1 positive donors at enrollment. However, the rate of fall of CD4 count (per month) and rate of increase in plasma viral load (per year), calculated on the basis of values at enrollment and that at one year post-enrollment follow up point were higher in the replacement category of donors compared to voluntary donors (Table 2).
In vitro production of IFN-γ by cultured PBMC: Levels of IFN-γ produced by PBMCs in response to mitogen PHA and to BCG (treated as well as untreated with rlL-12) were comparable to controls in both replacement and voluntary category of HIV-1 positive donors at both 1st and 2nd TST of baseline testing. However, IFN-γ production was depressed in response to PPD in case of replacement category of donors at 1st TST that showed significant augmentation in response to pre-treatment with rIL-12 at the same point of testing bringing it to level comparable to controls. At the point of second TST in two step testing in the same group of donors, IFN-γ production in response to PPD in PBMC culture was found to show normal level with or without pre-treatment with rIL-12 comparable to controls (Table 3).
Table 3: Mean ± SEM Levels of IFN-γ (μg mL-1) in supernatant of PBMC culture from HIV-1 positive blood donors stimulated with mitogen (PHA), M.tb specific antigen (PPD) and BCG with or without supplementation of recombinant 1L-12 (rIL-12). View Table 3
A total of 33 out of 68 (48.5%) donors in replacement category and 5 out of 71 (7.1%) of donors in voluntary category developed PT as the first presenting ARI on follow up (Relative risk 6.9; 95% CI 2.9-16.6; P < 0.001). Majority of PT cases in replacement donors were contributed by the subcategory that showed boosting/conversion phenomenon in two-step TST (28 out of 44 i.e. 63.6%) compared to PT cases contributed by the remaining donors in the same category available on follow up (5 out of 24 i.e. 20.8%, Relative risk 3.1; 95% CI 1.4-6.9; P < 0.001) (Figure 1). Symptom-free (PT-free) duration in donors developing PT on follow up negatively correlated with degree of change in TST reactivity between first and second TST among the donors in replacement category showing boosting (Figure 3). However, no such correlation could be observed in the same group of donors between degree of change in TST reactivity between 1st negative and 2nd positive TST at conversion (not shown in figure).
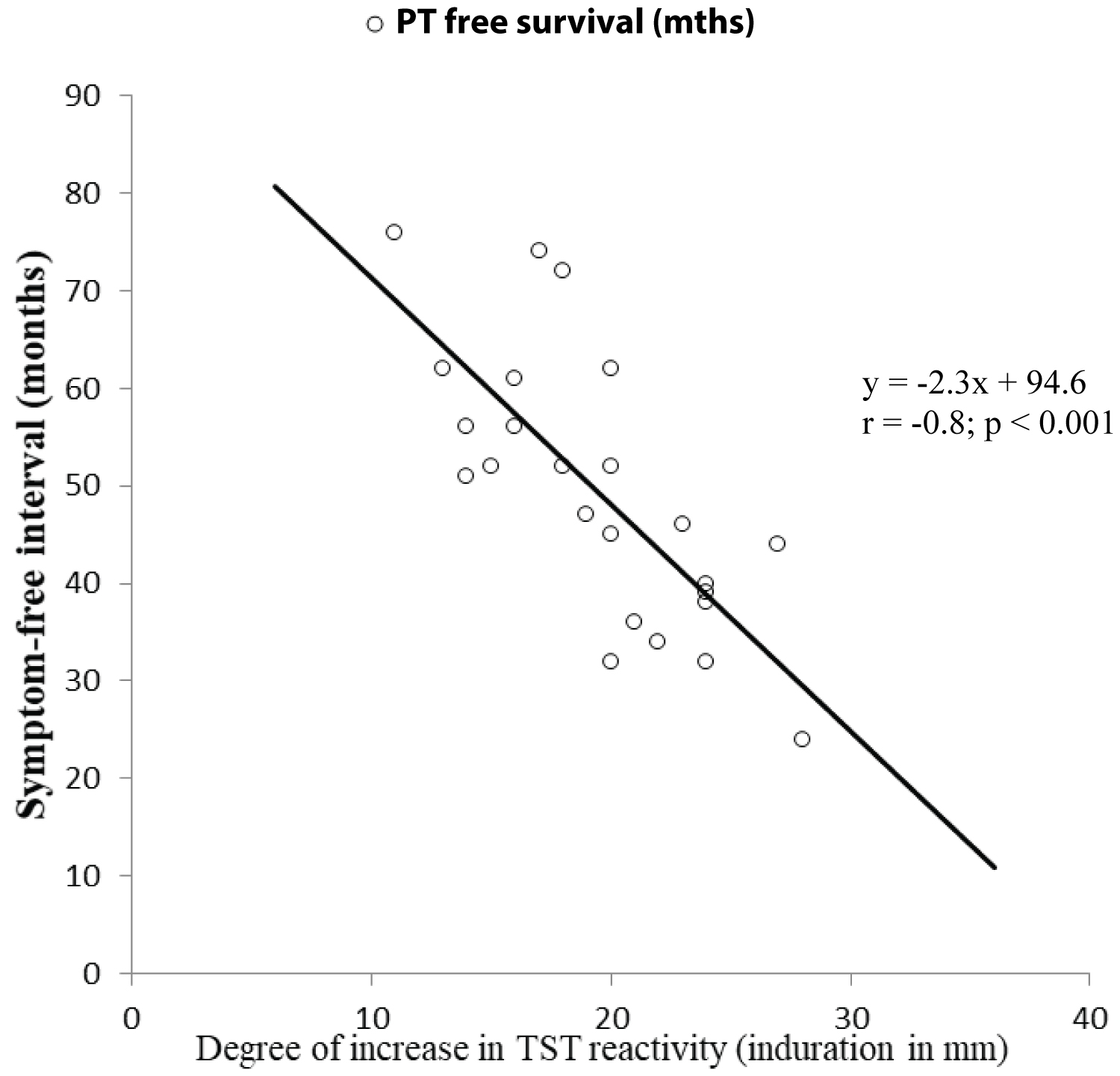 Figure 3. Correlation between the difference in degree of TST reactivity (induration diameter in millimeter) between 1st (negative) and 2nd (positive) TST in cases showing 'boosting' versus symptom free (PT free) interval in months.
View Figure 3
Figure 3. Correlation between the difference in degree of TST reactivity (induration diameter in millimeter) between 1st (negative) and 2nd (positive) TST in cases showing 'boosting' versus symptom free (PT free) interval in months.
View Figure 3
Evaluation of TST response towards prediction of disease progression in HIV-1 infected individuals has been mostly limited to subjects with established HIV-1 infection accompanied by varying degree of alterations in CD4 count [19-21]. However, information is limited on such evaluation in HIV-1 infected subjects at early asymptomatic stage with unimpaired CD4 counts.
In the present study the replacement category of donors were characterized by positive history of oral iron intake in many with biochemical evidence of iron overload at enrollment presumably due to excess intake of oral iron coupled with enhanced absorption due to concomitant intake of alcohol reported by the same category of donors [22]. Concern has been raised in national guidelines about the possibility that many of such donors categorized as replacement donors may actually be remunerated donors with false identity as relative or friend of the patient. Previous studies on remunerated blood donors during the pre-ban era in the country revealed highly prevalent practice of consumption of iron tablets in them as a precautionary measure to compensate for lowering of hemoglobin level due to high frequency of donation [6]. Return of the elevated iron parameters to normal levels at the point of second TST provided further indirect evidence of iron consumption by such donors reflecting possible discontinuation of iron consumption by them following diagnosis of HIV-1 infection due to their awareness of exclusion as future eligible donor by virtue of HIV-1 positivity.
The overall baseline TST positivity rate encountered in the HIV-1 positive donors (43.1%) in the present study is higher than that reported in India among the population with moderate risk of TB related exposure i.e. medical and nursing students (22%) [23] but is comparable to that reported among health care workers (41%) [24].
While there is no clear cut guidelines on interpretation for boosting reaction in TST among HIV infected individuals, few studies have adapted a definition of boosting as development of a TST with ≥ 5 mm induration after a previously negative test (< 5 mm) that was followed in the present study [25,26]. High incidence of boosting, almost exclusively confined to replacement donors as compared to voluntary donors, could not be related to difference in the CD4+ T cell counts (both the categories showing unaltered counts at enrollment by virtue of inclusion criteria) or nutritional status (serum albumin level as indicator) that were comparable between the two categories of donors. Moreover, proportions of donors with visible BCG immunization scars were comparable between the two categories of donors thus minimizing the possibility of confounding effect of prior BCG vaccination. Rather such observation could be related to suppression of TST response by high level of iron in blood detected at first TST and release from such suppressive effect due to normalization of iron levels by the time of second TST carried out after 2-3 weeks resulting from discontinuation of oral iron intake. Higher conversion rate in replacement category of donors at 1 year of post-enrollment follow up as observed in the present study could as well be a reflection of reversal of iron parameters since studies have indicated that conversion, particularly those within first two years of initial negative two-step testing could represent boosting rather than actual conversion [27,28]. However, such suppression of TST response was selective in nature against M.tb specific antigens alone without any associated effect on general immune status since simultaneous anergy testing in these donors employing recall antigens in multi-test CMI showed suppressed response against OT alone but demonstrated unimpaired response to other antigens included in the same anergy test panel. Selective impairment of immune response to mycobacterial antigens in replacement donors was further evident from the in vitro experiments in the present study where IFN-γ production by PBMC on stimulation by mitogen PHA in the same category was found to be comparable to that of control but was diminished in response to the M.tb specific antigens i.e. PPD. Moreover, such impaired production of IFN-γ was not accountable to any quantitative or intrinsic qualitative defect in the CD4+ T cells since the level of IFN-γ production could be restored on addition of rIL-12 in the same in vitro culture system in the present study. It has been shown that increasing IFN- γ secretion against Tb using prime boost strategy [29] or recombinant IFN-γ [30] did not enhance protection against challenge with M.tb suggesting contribution of factors in addition to IFN-γ for adequate lymphocyte induced macrophage killing of M.tb Several reports including one from our laboratory involving blood donors with iron overload suggested the role of macrophage induced IL-12 in efficient production of IFN-γ by the CD4+ T cells [31-33]. Unaltered response of PBMC to BCG in the face of impaired response to PPD further validates that the observed impaired TST response could be accepted as a reliable correlate of impaired response specific to M.tb comparable to other tests based on IFN-γ release assay using M.tb specific antigens like culture filtrate protein (CFP) or early secreted antigenic target (ESAT) e.g. Quantiferron Gold.
In the present study on HIV-1 infected donors, incidence of PT on follow up was many fold higher in the replacement category compared to voluntary donors. Relationship between iron overload and impaired nitric oxide mediated killing of M.tb leading to development of PT is well recognized [33,34]. Positive correlation between the serum ferritin level and increase in the diameter between first and second TST in replacement donors showing booster/conversion phenomenon suggests further the possible suppressive effect of ferritin on the M.tb specific immune response. Development of high incidence of PT in the same category of donors i.e. those showing boosting/conversion phenomenon despite normalization of iron parameters suggest a long term influence of iron overload on M.tb specific immunity rendering them prone to acquisition of M.tb infection from the community with high baseline prevalence of M.tb.
DTH testing for tuberculosis is not adequate for diagnosis of latent tuberculosis infection in population with high BCG coverage and high level of exposure to non-tubercular mycobacteria as in India. Nearly 40% of Indians are estimated to be latently infected [35]. Thus the Revised National Tuberculosis Control Program in India does not give priority to LTBI detection like many other developing countries. However findings in the present study point out the need to identify the subset of TST positive donors who would progress to develop TB and thus would be benefited by INH prophylaxis as strongly recommended by WHO [36]. Among the HIV-1 positive donors in our study, majority of the TST positive donors in replacement category with boosting/conversion developed PT compared to other groups of TST positive donors indicating that the former group of subjects would have derived most the benefit of WHO recommendation that was not considered in the country at the time of our study. A study by Giardi et al. showed that degree of TST reactivity correlated with subsequent incidence of tuberculosis in HIV-infected patients [37]. However, the present study relied on difference in TST reactivity between the 1st and 2nd steps of TST rather than one point TST reading in accordance with the observations by Garcia et al. and that by Huebner et al. who concluded that reversal of TST response in the two-step TST may be an independent predictor towards development of Tuberculosis in HIV infected non-anergic subjects with unimpaired CD4 count similar to the subjects included in our study [38,39]. However, the present study highlights a scenario of reversal of TST response consequent to normalization of iron overload masking the initial suppression of TST response that to the best of our knowledge is hitherto unreported.
The present paper provides evidence that iron overload may be responsible for false negative result in tuberculin skin testing (TST) corroborating with reduced interferon-gamma production by peripheral blood mononuclear cells stimulated with PPD containing M. tuberculosis specific antigen. Such false negative result may have implications in terms of timely initiation of anti-tubercular therapy and may lead to spread of infection to close contacts. Evidence of iron overload was limited to the replacement category of blood donors qualifying for donating blood with the identity as a close friend or relative of the patient. Thus, it is probable that this category of donors were engaged in frequent blood donation and consumption of iron pills to maintain hemoglobin level , a practice that was reported to be prevalent among remunerated blood donors with HIV related risk behaviors prior to the legal ban on remunerated blood donation in India. The present report supports the validity of the concern raised in national guideline for removing the replacement category of donors with questionable risk behavior from donor pool and switching over to 100% voluntary blood donation in the country.
The authors declare that there is no conflict of interests regarding the publication of this paper.