Breast cancer, a highly penetrant hereditary disorder, is the most common cancer in women worldwide. Approximately 10% of breast cancer cases are hereditary and 15% of patients with invasive breast cancer have a first-degree relative with the same disorder. Genetic counseling has become an important tool of the health care system providing information and support to families at risk of a genetic disorder. Oncology research teams have designed breast cancer screening guidelines for high-risk patients. Along the past decade, several genes have been identified as genetically related to breast cancer inheritance. BRCA1 and BRCA2 are considered the most important genes related to inheritance predisposition of breast cancer, along with PTEN and TP53 [1]. The BRCA1 and BRCA2 structures are different but their functions are interconnected and related to DNA repair. The susceptibility of breast cancer for patients with the BRCA1 mutation is up to 87% for older women. Another gene, TP53, codes for a protein that acts as the guardian of the genome, binds to DNA in order to perform transcriptional regulatory functions, regulation of the cell cycle and apoptosis among other functions. According to genetic counseling and epidemiologic studies, the risk of developing cancer for patients with TP53 polymorphisms is 90%. Regarding the gene PTEN, germline mutations increases the risk of breast cancer and about 80% of patients with breast cancer carry germline mutations in the gene. PTEN is an oncogene and codes for a protein with phosphatase activity, related to the regulation of cell cycle, controlling cells growth and able to promote cell cycle arrest. Currently, genetic counseling endeavor to identify patients at risk of genetic anomalies, study family history and inheritance patterns, calculate risks of recurrence, and provide information regarding testing and treatment procedures. Therefore, breast cancer patients and their families are presented with possibilities of screening for BRCA1, BRCA2, TP53, and PTEN mutations, and preventive care such as chemoprevention and prophylactic surgery.
Genetic Counseling, Polymorphisms, Breast Cancer, BRCA1, BRCA2, TP53, PTEN
Over the last decade, there were more than 500,000 cases of deaths from breast cancer in the world [2]. Breast cancer is the most common cancer in women worldwide. It has been investigated for more than 2000 years throughout history and its relation to a genetic component was described in 1700 in a young female with breast cancer whose maternal uncle and grandmother had died of the same type of cancer [3].
Breast cancer is a highly penetrant hereditary disorder. Germinal mutations occur in cancer susceptibility genes and is inherited either from the paternal or maternal origin. Approximately 10% of breast cancer cases are hereditary [4] and 15% of patients with invasive breast cancer have a first-degree relative with the same disorder [5]. Genetic counseling has become an important tool of the health care system providing information and support to families at risk of a genetic disorder. Oncology research teams have designed breast cancer screening guidelines for high-risk patients [6-9].
Genetic counseling can lead to the detection of early-stage breast cancer tumors. In Europe, for example, breast cancer screening reduced patient mortality by 48% in patients during the first decade of the twenty-first century [10]. In Italy, breast cancer screening let to a significant survival rate for patients after 5 years from the date of diagnosis [11]. A positive family history for breast cancer is one of the strongest predictors of a patient's risk of developing the disease [12]. Therefore, genetic counseling is routinely provided to patients with increased risk of carrying genetic mutations [13].
Along the past decade, several genes have been identified as genetically related to breast cancer inheritance. The most studied ones are ATM (Ataxia Telangiectasia-Mutated) [14], BRCA1 (Breast cancer gene 1), BRCA2 (Breast Cancer gene 2) [15], BRIP1 (BRCA1 interacting protein C-terminal helicase 1) [16], CHEK2 (Checkpoint kinase 2) [17], PALB2 (Partner and localizer of BRCA2) [18], PTEN (Phosphatase and tensin homolog) [19], TP53 (Tumor Protein p53) [1] and RAD51C (RAD51 paralog C) [20]. In the present review, we explore the involvement of high-penetrance genes (BRAC1, BRCA2, TP53 and PTEN) with breast cancer, familial history of the disease and genetic counseling.
The molecular and genetic basis of inherited breast cancer risk started to be unraveled after the discovery of BRCA1 [21,22] and BRCA2 [23] in the 90's. Currently, BRCA1 and BRCA2 [24] are considered the most important genes related to inheritance predisposition of breast cancer, along with PTEN [19] and TP53 [1]. BRAC1 and BRAC2 are oncogenes, also known as caretaker genes, responsible for the error-free repair and maintenance of genomic stability through a homologous recombination process [15].
The BRCA1 gene codes for a protein (BRAC1) that interact with other proteins such as tumor suppressors, DNA damage sensitive proteins and cell signaling protein (Figure 1). The multiprotein complex formed is so-called the BRCA1-associated genome surveillance complex (BASC) [25]. Moreover, BRCA1 interacts with RNA polymerase and histones in order to drive transcriptional and transcriptional regulation [26], cell cycle progression and ubiquitination [27]. The BRCA1 protein contains two main domains. A zinc finger domain related to its ubiquitination function [28] and a BRCA1 C Terminus domain (BRCT) related to its DNA repair function [29].
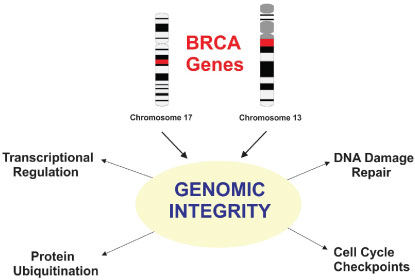 Figure 1: Schematic view of BRCA1 (chromosome 17) and BRCA2 (chromosome 13) genes and their functions to maintain genomic stability through transcriptional regulation, protein ubiquitination, cell cycle checkpoints and DNA repair. View Figure 1
Figure 1: Schematic view of BRCA1 (chromosome 17) and BRCA2 (chromosome 13) genes and their functions to maintain genomic stability through transcriptional regulation, protein ubiquitination, cell cycle checkpoints and DNA repair. View Figure 1
The BRCA1 and BRCA2 structures are different but their functions are interconnected and related to DNA repair. BRCA2 is key to homologous recombination as its protein takes part in a nucleoprotein complex in order to identify regions of homology in the DNA, recruit partners such as RAD51C and PALB2 [30,31], repair strand breaks and maintain genome stability. BRCA2 has an important role in meiosis, a process of cell division that produces sperm and eggs. During meiosis, chromosomes exchange genetic material and are prone to undergo DNA strand break and BRCA2 is essential for fixing such damage in the DNA. Studies performed in mice have shown that individuals with mutations in BRCA2 have serious growth problems, are sterile and fail to produce spermatozoa [32].
Breast cancer incidence and death rates increase with age. The cumulative incidence of breast cancer is 0.4% and although the disease is not very common in young patients, it is the most frequent type of cancer in women from 15 to 40-years-old [33]. The susceptibility of breast cancer for patients with the BRCA1 mutation is up to 87% for women that are over 70-years-old, with little variation among ethnic groups [34]. Genetic counseling and cancer susceptibility evaluation are of great significance for the diagnosis of breast cancer patients. BRCA1 and BRCA2 mutation screening is performed for at-risk families through genetic counseling. Patients and their family need to comprehend the significance of the counseling in order to make decisions regarding prevention strategies. This way, the outcome of genetic counseling is able to guide clinical decisions and disease management [35].
The TP53 gene has a sequence that is highly conserved across species [36]. The protein p53 acts as the guardian of the genome, binds to DNA in order to perform transcriptional regulatory functions [37], regulation of the cell cycle, apoptosis [38], inhibition of angiogenesis [39] and maintaining genome integrity [40] (Figure 2). TP53 becomes induced as a response not only to DNA damage, but also oxidative stress, osmotic shock, ribonucleotide levels reduction, and anomaly regarding expression of tumor suppressor genes [41]. TP53 is a tumor suppressor, preventing tumorigenesis [42]. Mutations on the p53 DNA binding motif is an indication of cancer susceptibility and should be investigated through genetic counseling [43]. TP53 is the most frequently mutated gene in cancer. It is related to familial breast cancer, especially when it involves germinal mutations [44].
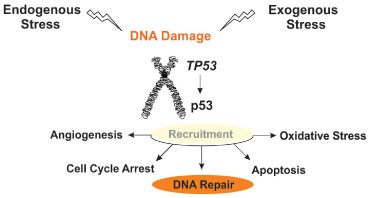 Figure 2: Schematic view of the TP53 gene functions. The protein p53 when expressed at normal levels recruits partner and regulates several processes that regulate oncogenesis, such as angiogenesis, cell cycle arrest or progression, apoptosis, and oxidative stress. TP53 is activated both by endogenous or exogenous signals. View Figure 2
Figure 2: Schematic view of the TP53 gene functions. The protein p53 when expressed at normal levels recruits partner and regulates several processes that regulate oncogenesis, such as angiogenesis, cell cycle arrest or progression, apoptosis, and oxidative stress. TP53 is activated both by endogenous or exogenous signals. View Figure 2
Polymorphic genes, especially oncogenes, increase the susceptibility to cancer. There is a common polymorphism regarding the TP53 gene, characterized by the substitution of the amino acid arginine for a proline at codon 72. Innumerous studies have tried to find a link between TP53 polymorphism and diseases [45-47], including cancer [48-51]. Alternative methods of cancer treatment have been applied, such as the exogenous p53 transduction [52,53] to increase the levels of the protein and restoration of endogenous wild-type p53 activity premature aging [54]. The former alternative shows some downsides such as premature aging and the latter show promising results since it promotes tumor cells remission without harming surrounding cells and preventing metastases. After extensive investigation, methods such those could be indicated by genetic counselors in order to prevent diseases.
Genetic counseling families at risk of breast cancer due to mutation on TP53 is never a simple task due to the wide likelihood of the clinical anomalies related to it. Moreover, the idiopathic causes may lie on association with other tumor suppressor genes [55]. According to genetic counseling and epidemiologic studies, the risk of developing cancer for patients with TP53 polymorphisms is 90%, and breast cancer is included in that estimative [56]. Women show up to 85% risk of developing breast cancer before they reach the 45 years of age [55,56]. In addition, 20% of TP53 mutation related tumors occur before the age of 20 years [55]. The complexity of TP53 related cancers may bring psychosocial issues for the affected families and genetic counseling may help them with decisions regarding prevention strategies.
PTEN is an oncogene and codes for a protein with phosphatase activity, related to the regulation of cell cycle, controlling cells growth and able to promote cell cycle arrest [57] (Figure 3). PTEN genetic alterations may lead to tumor onset due to loss of its enzyme product and inability to control cell mitosis and at the same time, those cells show a reduced apoptosis rate. Phosphatidylinositol triphosphate (PIP3) is the substrate of the protein coded by PTEN. PIP3 is a signal molecule during tumorigenesis that takes part in cascade pathways related to cell growth and invasion [58]. PTEN regulates growth down-regulating PIP3 intracellular levels, thus, avoiding growth and invasion of cells that lost control over mitosis [59,60].
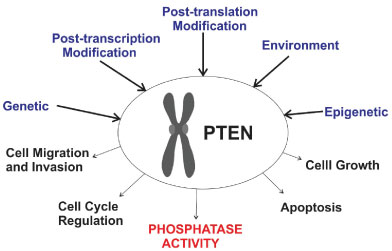 Figure 3: Schematic view of factors that may change PTEN expression and alter its functions, consequently leading to a possible tumor cell onset. View Figure 3
Figure 3: Schematic view of factors that may change PTEN expression and alter its functions, consequently leading to a possible tumor cell onset. View Figure 3
Genetic anomalies related to PTEN is found in glioblastoma [61], endometrial [62], prostate [63,64], lung [65,66] and breast cancer [67-69]. Several studies have shown that PTEN is related to the onset and development of breast cancer. The underlying mechanisms involved in breast cancer tumorigenesis due to PTEN mutations include a large variety of genomic processes. The most common ones are germline [70] and somatic mutations [71], epigenetic regulation [72], PTEN protein-protein interactions [73], PTEN protein degradation and post-translational modifications [74,75] that causes repressed expression of the PTEN protein and loss of heterozygosity [76].
Assessment of family history helps to determine the risk of breast cancer. It has been shown that germline mutations in PTEN increases the risk of breast cancer. About 80% of patients with breast cancer carry germline mutations in PTEN [77]. In addition, the rate of PTEN deletion is 40% and the polymorphisms within PTEN sequence is less than 5% [78]. Breast cancer patients with loss of at least one allele of the PTEN gene show very poor prognosis [76,79]. Women with mutation in the PTEN gene have up to 76% risk for benign breast anomalies [80]. Interestingly, males with genetic disorders in PTEN have been diagnosed with breast cancer [77,81].
Genetic counseling is recommended for families with historical cases of PTEN anomalies and breast cancer. PTEN anomalies is clinically assessed by criteria developed by the International Cowden Consortium in 1995 and the National Comprehensive Cancer Network Genetics/High Risk Cancer Surveillance Panel [82]. Genetic counselors normally suggest that women with historical family of PTEN breast cancer should breast self-exam every month and clinical breast examinations should be performed semiannually. In addition, clinical mammography should be performed annually [82,83].
Here, we explored the involvement of high-penetrance genes (BRAC1, BRCA2, TP53 and PTEN) with breast cancer, familial history of the disease and genetic counseling. BRAC1 and BRAC2 are genome caretaker genes, responsible for error-free repair and maintenance of genomic stability. BRCA1 and BRCA2 mutation screening should be performed for at-risk families through genetic counseling and clinical assessment, since mutations affecting them account to a large number of breast cancer. Mutations on the p53 DNA binding motif increase cancer susceptibility and should also be investigated through genetic counseling. Germline mutations in PTEN increases the risk of breast cancer and women with family history of the disease show 50% risk of developing breast cancer and 80% of them carry germline mutations in PTEN.
The number of patients with family history of breast cancer engaging genetic counseling is increasing. This should lead an improvement of breast cancer surveillance and a more effective preventive care. Currently, genetic counseling endeavor to identify patients at risk of genetic anomalies, study family history and inheritance patterns, calculate risks of recurrence, and provide information regarding testing and treatment procedures. Therefore, breast cancer patients and their families are presented with possibilities of screening for BRCA1, BRCA2, TP53, and
PTEN
mutations, and preventive care such as chemoprevention and prophylactic surgery.The author declares no financial support was sought in this project and there is no conflicts of interest.