Uterine sarcomas comprise a group of rare tumours with differing tumour pathobiology, natural history and response to clinical treatment. Diagnosis is often made following surgical treatment for presumed malignant mesenchymal tumours and benign tumours. Currently pre-operative diagnosis does not reliably distinguish between malignant mesenchymal tumours, Uterine Leiomyosarcoma (U-LMS) and benign tumours including Leiomyomas (LMA). U-LMS is the most common sarcoma but other subtypes include endometrial stromal sarcoma (low grade and high grade), undifferentiated uterine sarcoma and adeno sarcoma. Clinical trials have shown no definite survival benefit for adjuvant radiotherapy or chemotherapy, and have been hampered by the rarity and heterogeneity of these tumour types. There is a role of adjuvant treatment in carefully selected cases following multidisciplinary discussion at U-LMS reference centres. In patients with metastatic LMS then systemic chemotherapy can be considered. Accordingly, it is necessary to analyse risk factors associated with human U-LMS, in order to establish a treatment method. Proteasome β-subunit 9 (PSMB9)/β1i-deficient mice spontaneously develop U-LMS, with a disease prevalence of ~37% by 12 months of age. We found PSMB9/β1i expression to be absent in human U-LMS, but present in human LMA. Therefore, defective PSMB9/β1i expression may be one of the risk factors for human U-LMS. PSMB9/β1i is a potential diagnostic-biomarker for human U-LMS, and may be targeted-molecule for a new therapeutic approach.
PSMB9/β1i, Diagnosis, Mesenchymal tumour, Leiomyosarcoma, Leiomyoma
U-LMS: Uterine Leiomyosarcoma; LMA: Leiomyomas; SEER: Surveillance Epidemiology and End Results; PSMB9: Proteasome β-subunit 9; IHC: Immunohistochemistry
Soft tissue sarcomas arising from the uterus are a rare and varied group of neoplasms all of mesenchymal origin. They can occur at any anatomical site and exhibit a wide range of behaviours which largely depend on the histologic subtype and associated tumour grade. The incidence of human Uterine Leiomyosarcoma (U-LMS) is around 3-7% of all uterine malignancies and is associated with a poor prognosis when compared to endometrial carcinoma [1]. Recent results from Surveillance Epidemiology and End Results (SEER) database analysis have shown a higher incidence rate for those aged 40 years or older, compared to younger patients and twice the incidence in women of Afro-Caribbean descent compared to Caucasian women [2].
The development of gynaecologic tumours is often correlated with female hormone secretions; however, the development of human U-LMS is not substantially correlated with hormonal conditions, and the risk factors are not yet known. Importantly, a diagnostic-biomarker which distinguishes malignant human U-LMS from other uterine mesenchymal tumours including benign tumour Leiomyoma (LMA) is yet to be established. Accordingly, it is necessary to analyze risk factors associated with human U-LMS, in order to establish a treatment method. Proteasome β-subunit 9 (PSMB9)/β1i-deficient mice spontaneously develop U-LMS, with a disease prevalence of ~37% by 12 months of age. We found PSMB9/β1i expression to be absent in human U-LMS, but present in human LMA. Therefore, defective PSMB9/β1i expression may be one of the risk factors for human U-LMS. PSMB9/β1i is a potential diagnostic-biomarker for human U-LMS, and may be targeted-molecule for a new therapeutic approach.
Takuma Hayashi (Shinshu University School of Medicine) discussed a novel biomarker for detecting human U-LMS. Patients with U-LMS typically present with vaginal bleeding, pain and a pelvic mass, with atypical presentations of hypercalcemia and eosinophilia also being reported [3-5]. There is concern that radiographic evaluation with combined positron emission tomography/computed tomography, which is commonly used to aid assessment of patient prognosis, might not necessarily be effective for diagnosis and surveillance of human U-LMS [6-8]. Unfortunately, radiographic imaging cannot provide any medical information to help distinguish between U-LMS and other uterine mesenchymal tumours including benign tumour LMA [9,10].
Importantly, diagnostic biomarkers that are able to distinguish between human U-LMS and other uterine mesenchymal tumours are not yet established. In eukaryotes, proteasomes are large, ATP-dependent complexes located in both nucleus and cytosol. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. The proteasome and its subunits are of clinical significance, a compromised complex assembly or a dysfunctional proteasome can be associated with the underlying pathophysiology of specific diseases. Hayashi's research group reports that PSMB9/β1i-deficient mice exhibit spontaneous development of human U-LMS, with a disease prevalence of ~37% by 12 months of age [11]. The current focus of Hayashi's research is to probe the loss of PSMB9/β1i expression in human U-LMS, as well as the detectable expression of the protein in human LMA [12,13]. Defective expression of PSMB9/β1i is likely to be one of the risk factors for the development of human uterine neoplasms, as it is in the PSMB9-deficient mouse. Thus, PSMB9/β1i is useful as a novel diagnostic biomarker for human U-LMS, and Hayashi's research group have been trying to establish a novel diagnostic biomarker with PSMB9/β1i, which can distinguish the human U-LMS from other human uterine mesenchymal tumours including LMA under the SIGMA-Aldrich Collaboration Laboratory Project.
The Immunohistochemistry (IHC) experiments, performed separately at several medical facilities, revealed a serious loss in the ability to induce the expression of PSMB9/β1i in human U-LMS tissues in comparison with normal human myometrium tissues located in same tissue sections: normal myometrium; total 74 cases, uterine LMA; total 52 cases, Bizarre LMA; total 3 cases, U-LMS; total 58 cases. Of the 58 patients with U-LMS that we examined, 49 were negative for PSMB9/β1i expression, 4 were focally positive, and 3 were partially positive. Two patients with U-LMS were analyzed for the expression of PSMB9/β1i [12,13]. PSMB9/β1i levels were also evaluated in skeletal muscle and rectal metastases from individual patients with U-LMS, where surgical samples showed the presence of a mass measuring 3 cm in maximum diameter in the lumbar quadrate muscle without a fibrous capsule. All lymph nodes were negative for human LMS metastases, and IHC analyses with U-LMS showed positivity for Ki-67 (clone MIB1) and negativity for PSMB9/β1i (Figure 1 and Table 1). The defective expression of PSMB9/β1i detected in primary human U-LMS was also observed in the metastatic LMS of the skeletal muscle and rectum, indicating that the metastatic lesions conserved this biological characteristic of primary human U-LMS. In both western blotting and RT-PCR experiments, PSMB9/β1i was expressed in normal myometrium but not in human U-LMS, which was strongly supportive of the IHC findings. Furthermore, analysis of a human U-LMS cell line clarified the biological significance of PSMB9/β1i in malignant myometrium transformation and cell cycle, thus implicating PSMB9/β1i as an anti-tumorigenic candidate [12,13]. Human U-LMS reportedly is a highly metastatic smooth muscle neoplasm for which CALPONIN h1 is suspected to have a biological role as a tumor suppressor. Moreover, several lines of evidence indicate that although CALPONIN h1 does not directly influence tumorigenesis, it clearly affects PSMB9/β1i-induced cellular morphological changes [14].
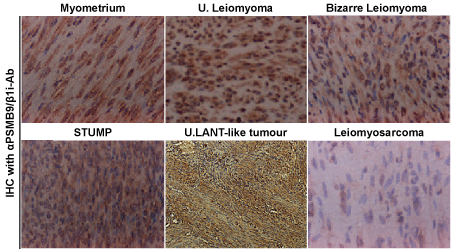 Figure 1: Differential expression of PSMB9/β1i in human normal myometrium, several mesenchymal tumour types and uterine LANT-like malignant tumour. Immunohistchemistry (IHC) of PSMB9/β1i in normal myometrium, usual leiomyoma, Bizarre leiomyoma, Smooth Muscle Tumour of Uncertain Malignant Potential (STUMP) and Uterine Leiomyosarcoma (LMS) tissues located in same tissue, and uterine LANT-like malignant tumour. For all samples, 5-µm sections of tissues specimens were stained with anti-PSMB9/β1i antibody revealed by peroxidase-conjugated anti-rabbit IgG antibody.
View Figure 1
Figure 1: Differential expression of PSMB9/β1i in human normal myometrium, several mesenchymal tumour types and uterine LANT-like malignant tumour. Immunohistchemistry (IHC) of PSMB9/β1i in normal myometrium, usual leiomyoma, Bizarre leiomyoma, Smooth Muscle Tumour of Uncertain Malignant Potential (STUMP) and Uterine Leiomyosarcoma (LMS) tissues located in same tissue, and uterine LANT-like malignant tumour. For all samples, 5-µm sections of tissues specimens were stained with anti-PSMB9/β1i antibody revealed by peroxidase-conjugated anti-rabbit IgG antibody.
View Figure 1
Table 1: Differential expressions of PSMB9/β1i and Ki-67/MIB1 in human uterine mesenchymal tumours and uterine LANT-like tumour. View Table 1
There is only one prospective Phase II study of aromatase inhibitors in human U-LMS with primary endpoint of Progression-Free Survival (PFS) at 12 weeks [15]. Whilst no objective responses were seen stable disease was recorded in 14 out of 27 patients and PFS at 12 weeks of 50%, and median duration of treatment only 2.2 months. This therapeutic approach has the benefit of being relatively well tolerated, especially when compared to chemotherapy and is recommended in patients with more indolent disease patterns and lower burden of disease. The physiological significance of PSMB9/β1i as a tumour suppressor may lead to new therapeutic targets in human U-LMS.
Defective expression of PSMB9/β1i is likely to be one of the risk factors for the development of human uterine neoplasm, as it is in the PSMB9/β1i deficient mouse. Thus, combination of PSMB9/β1i with other functional candidates is useful for a novel diagnostic biomarker for human U-LMS [16-19]. Additionally, gene therapy with PSMB9/β1i expression vectors may be a new treatment for human U-LMS that exhibit a defect in PSMB9/β1i expression. Because there is no effective therapy for unrespectable human U-LMS, our results may bring us to specific molecular therapies to treat this disease.
Immunohistochemistry (IHC) was performed using the avidin-biotin complex method previously described [19]. Briefly, one representative 5-µm tissue section was cut from a paraffin-embedded sample of the radical hysterectomy specimen from 58 patients with uterine Leiomyosarcoma (LMS), 52 patients with Leiomyoma (LMA), 3 patients with Bizarre LMA, 1 patient with Leiomyomatoid Angiomatous Neuroendocrine Tumour (LANT)-like tumour, normal myometrium total 74 cases. Sections were deparaffinized and rehydrated in graded alcohols and then incubated with normal mouse serum for 20 min. Sections were incubated at room temperature for 1 hour with anti-human PSMB9/β1i antibody and anti-Ki-67/MIB1 antibody (SIGMA-Aldrich Israel, Rehovot, Israel., dilution 1/100). Afterwards, sections were incubated with a biotinylated secondary antibody (Dako, Carpinteria, CA) and then exposed to a streptavidin complex (Dako). Complete reaction was revealed by 3,3'-diaminobenzidine, and the slide was counterstained with hematoxylin. Twenty one normal human myometrium sections from specimens were used as positive controls. Negative controls consisted of tissue sections also incubated with normal rabbit IgG instead of the primary antibody. These studies are registered, at Shinshu University in accordance with local guidelines (approval no. M192).
We sincerely appreciate the generous donation of PSMB9/β1i-deficient breeding mice and technical comments by Dr. Luc Van Kaer (Vanderbilt University Medical School, Nashville, Tennessee). We thank Isamu Ishiwata for his generous gift of the human U-LMS cell lines. We appreciate the technical assistance of the research staff at Harvard Medical School. We are grateful to Dr. Tamotsu Sudo and Dr. Ryuichiro Nishimura (Hyogo Cancer Centre, Akashi, Hyogo, Japan) for their generous assistance with Immunohistochemistry (IHC) analysis and helpful discussion. This work was supported by grants from the Ministry of Education, Culture, Science and Technology, the Japan Science and Technology Agency (JST), the Foundation for the Promotion of Cancer Research, Kanzawa Medical Research Foundation, and The Ichiro Kanehara Foundation.