International Journal of Clinical Cardiology
Serum Omentin-1 Concentrations and Biochemical Markers of Chronic Subclinical Inflammation in Obese Subjects
Eman M Alissa1*, Maisa'a M Al-Salmi1, Nabeela Alama1 and Gordon A Ferns2
1Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
2Medical Education and Metabolic Medicine, Brighton and Sussex Medical School, University of Brighton, BN1 9PH, UK
*Corresponding author: Eman M Alissa, Faculty of Medicine, King Abdulaziz University, PO Box 12713, Jeddah 21483, Kingdom of Saudi Arabia, Tel: (966) 2 6400000, Fax: (966) 2 6643499, E-mail: em_alissa@yahoo.com
Int J Clin Cardiol, IJCC-2-061, (Volume 2, Issue 6), Original Research; ISSN: 2378-2951
Received: September 17, 2015 | Accepted: November 18, 2015 | Published: November 21, 2015
Citation: Alissa EM, Al-Salmi MM, Alama N, Ferns GA (2015) Serum Omentin-1 Concentrations and Biochemical Markers of Chronic Subclinical Inflammation in Obese Subjects. Int J Clin Cardiol 2:061. 10.23937/2378-2951/1410061
Copyright: © 2015 Alissa EM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Whilst chronic subclinical inflammation is now considered to be a predisposing risk factor of cardiovascular diseases. The extent by which adipokines induce metabolic abnormalities in humans is not fully resolved. The purpose of this study was to examine the relationship between insulin resistance and serum inflammatory markers in obese subjects.
Methods: One hundred and five subjects without any clinically evident CVD were classified into 3 coronary risk levels according to Framingham risk score. Demographic and anthropometric variables were estimated. Serum levels of lipid profile, blood glucose, insulin, omentin-1 and high sensitivity-Reactive Protein (hs-CRP) were measured in fasting blood samples. Insulin resistance indices were also calculated.
Results: 29% and 62% of the study population were overweight and obese respectively by Body mass index (BMI) measures. Almost half of the study population was considered diabetic. There was a tendency for a fall in serum omentin-1 concentrations with increasing coronary risk with a significant increase in hs-CRP levels in the same direction (p < 0.05). Age and fasting blood glucose were found to be independently associated with serum omentin-1 levels. BMI and fasting blood glucose were independent determinants of serum hs-CRP levels.
Conclusions: Omentin-1 might be associated with the development of diabetes mellitus indirectly via insulin activity and obesity. These findings may have important implications for the pathophysiology and therapy of diabetes mellitus by further longitudinal studies.
Keywords
Omentin-1, hs-CRP, Subclinical inflammation, Insulin resistance, Diabetes mellitus, Obesity
Introduction
Adipose tissue is a dispersed endocrine organ, and a source of several hormones, for example leptin, and a number of cytokines known to be involved in systemic inflammation; these include plasminogen activator inhibitor type 1 (PAI-1), interleukin 6 (IL-6), and tumor necrosis factor-α (TNF-α) [1].
Cardiovascular disease (CVD) is now recognized to be a process involving inflammatory processes, and serum inflammatory markers are considered to be important for the evaluation of cardiovascular risk [2].
It is possible that the relationship between CVD risk and obesity are linked by the increased inflammatory milieu [3]. Obesity related inflammation has also been proposed as a possible mechanism by which obesity increases insulin resistance and leads to diabetes [4].
Whilst chronic subclinical inflammation is now considered to be a predisposing risk factor of CVD [5]. The extent by which adipokines induce metabolic abnormalities in humans is not fully resolved [6].
Omentin-1 is a circulating adipokine that is down-regulated in patients with CVD. Decreased omentin expression was shown to be implicated in a variety of chronic inflammatory diseases [7,8], and has been identified as an adipokine that may improve insulin sensitivity [9], although its circulating levels in obesity have not been adequately studied and its correlation with insulin resistance or obesity is still controversial.
We hypothesized that insulin resistance in obese subjects is associated with higher serum concentrations of inflammatory cytokines and that the association of cytokines with insulin sensitivity may be independent of body fat mass.
The purpose of this study was to examine the relationship between insulin resistance and serum inflammatory markers in obese subjects.
Methods
Subjects
One hundred and five subjects, attending the outpatient clinic in King Abdulaziz University Hospital, who were without any clinically evident CVD were considered for inclusion in this cross-sectional study. All patients gave their written informed consent for participation in the study and the ethics committee at KAUH approved the study protocol.
Exclusion criteria included subjects with liver, kidney, thyroid, malignancy, acute, or chronic infectious or inflammatory diseases. Subjects taking medications, such as, statins and aspirin that could affect inflammatory markers levels were also excluded from the study.
The Framingham risk score used in this study is a version defined in the ATP III report and is a composite score of traditional cardiovascular risk factors that includes age and sex, systolic blood pressure, total cholesterol, HDL-C, presence of diabetes, and smoking status [10].
Anthropometric measurements
Clinical assessment included anthropometric measurements and blood pressure readings. Data on health status were obtained from medical files and supplemented by the participants' self-reported health-related data. Body weight, height, body mass index (BMI), waist circumference, hip circumference, and waist to hip circumference ratio (WHR) were estimated for all study subjects. Body height and weight were measured using a stadiometer and a standardized balance-beam scale, respectively. Waist circumference was measured at the level of the umbilicus with silent breathing and hip circumference was measured at the inter-trochanteric girth according to the WHO guideline [11] in standing position. BMI was calculated as weight (kg) divided by height (m2) and WHR was obtained from waist circumference divided by hip circumference. Gender-based waist circumference and WHR cutoffs were employed as a measure of cardiovascular risk [12].
Blood pressure measurements were obtained on each subject following a 10 minute rest period in a seated position using auscultation and a mercury sphygmomanometer. The average of three successive readings of systolic and diastolic pressure was used as the documented blood pressure values.
Biochemical tests
Blood samples were collected from all participants after a 12 hour overnight fasting into plain and EDTA tubes. Fasting blood glucose levels were measured using an automated analyzer (Dimension Vista System, Siemens, Germany) standard enzymatic method. Fasting insulin was measured using an enzyme amplified chemiluminescence assay (Modular E170 immunoassay analyzer, Roche, USA).
The homeostasis model assessment of insulin resistance (HOMA-IR) and the homeostasis model assessment of β-cell insulin secretion (HOMA-IS) were calculated from fasting insulin and glucose levels using the following equations: HOMA-IR = Fasting insulin (mU/L) × Fasting blood glucose (mmol/L)/22.5; HOMA-IS = [20 × Fasting Insulin (mU/L)]/[Fasting blood glucose (mmol/L) - 3.5] [13]. Quantitative insulin sensitivity check index (QUICKI) was calculated by (QUICKI = 1/[log (fasting insulin) + log (fasting glucose)] [14].
A residual aliquot of serum from the fasting blood sample on each participant was stored at -80 °C. Serum high sensitive C-reactive protein (hs-CRP) was measured by means of immuno turbidimetric assay (Behring Nephelometer-BNA2, Siemens, USA). Three categories of serum hs-CRP were defined based on the cut-off points for coronary risk stratification, < 1.0 mg/L is considered a low risk, between 1.0 and 2.99 mg/L an intermediate risk, and ≥ 3.0 mg/L a high risk [15].
Serum omentin-1 was measured by ELISA in duplicate using commercially available kits (Biovendor, Germany). The intra-assay CV was 3.2% and inter-assay CV was 4.4%.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical variables were expressed as percentage. Kolmogorov-Smirnov test was performed to verify the normal distribution of the data. Logarithmically transformed values were used for the statistical analysis.
ANOVA test for normally distributed parameters or Kruskal-Wallis test for non-normally distributed parameters were used to compare mean values of continuous variables in between the subgroups followed by Bonferroni's test. Categorical variables were compared by χ2 or Fisher exact tests as appropriate. Correlations between continuous variables were assessed with the use of Pearson correlation test or Spearman correlation rank test as appropriate. Multiple stepwise regression analysis was performed to determine significant confounding factors for serum hs-CRP and omentin-1 levels. P values < 0.05 were considered to be statistically significant. All the statistical analyses were performed using SPSS version 16 software (SPSS Inc., Chicago, IL, USA).
Results
The study cohort consisted of 105 participants, aged 40 to 78 years, 73% of whom were females and 27% were males. Ten percent were lean, 29% were overweight, and 62% were obese according to their respective BMI classes namely, 18.5-24.9 kg/m2; 25-29.9 kg/m2; and ≥ 30 kg/m2. Approximately 66% of the male subjects had a waist circumference > 102 cm in comparison with 95% of the female subjects with waist circumference > 88 cm. Alternatively, 43% of males had WHR ≥ 0.95 where as 97% of females had WHR ≥ 0.80.
In further analysis the patients were divided into those with low coronary risk (n = 62), those with intermediate coronary risk (n = 25), and those with high coronary risk (n = 18) based on their Framingham score of 10 year CVD risk.
Table 1 provides clinical characteristics of the study participants. Those with low coronary risk had significantly lower body height and WHR values than those with high coronary risk (p < 0.05). Additionally, significant differences in age and blood pressure readings existed between the low coronary risk groups versus the remaining groups (p < 0.05).
![]()
Table 1: Clinical characteristics of the study participants (N = 105).
View Table 1
Although no significant differences in mean levels of fasting blood glucose, fasting insulin, and/or insulin resistance indices were found across the subgroups, 44% of the study participants were considered diabetic with fasting blood glucose ≥ 7 mmol/L (Table 2).
![]()
Table 2 Correlation analysis of measures of body fat and insulin resistance with serum hs-CRP and omentin-1 levels in the study participants (N = 105). Significant correlations are shown in bold font.
View Table 2
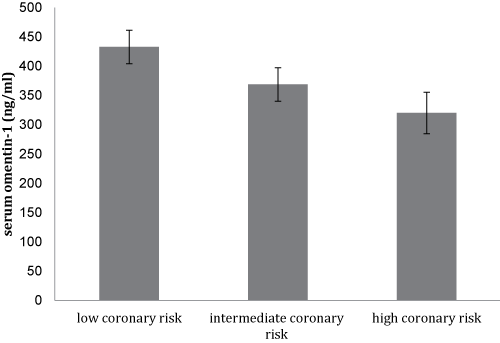
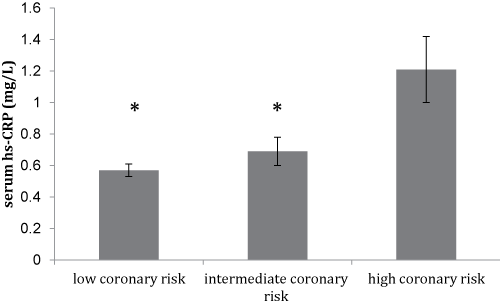
Notably, there was a trend for a lower mean serum omenin-1 concentration with the increasing coronary risk as shown in figure 1a, though this did not reach statistical significance. Furthermore, this was accompanied by a significant increase in serum hs-CRP levels (p < 0.05) between subjects with low, intermediate, and high coronary risk (Figure 1b).
.
Figure 1A: Mean values ± SD of serum omentin-1 levels of the study participants (N = 105) stratified by their Framingham scores of 10-year CVD risk.
View Figure 1A
.
Figure 1B: Mean values ± SD of serum hs-CRP levels of the study participants (N = 105) stratified by their Framingham scores of 10-year CVD risk. (*p < 0.05 vs. high coronary risk group)
View Figure 1B
Table 2 shows correlation analysis of the measures of body fat and insulin resistance with serum hs-CRP and omentin-1 levels. There were significant associations between circulating omentin-1 levels and age, fasting blood glucose, HOMA-IR, and QUICK-I. However, there were no association between serum hs-CRP and omentin-1 levels. Serum hs-CRP levels were significantly associated with anthropometric measurements including body weight, BMI, waist and hip circumferences, and fasting blood glucose.
When multiple regression analysis was performed to determine which variables were independently associated with serum omentin-1 levels, age and fasting blood glucose remained significant (Table 3). Table 4 shows that BMI and fasting blood glucose were independent determinants of serum hs-CRP levels.
![]()
Table 3 Multiple regression analysis of omentin-1
View Table 3
![]()
Table 4 Multiple regression analysis of hs-CRP
View Table 4
Discussion
Several adipokines have been proposed to be linked directly to insulin resistance and obesity [1]. Measurement of their serum levels may be an early marker for atherosclerosis risk. Serum hs-CRP levels have been used as a marker of subclinical inflammation which is expected to identify subjects at early stages of CVD [16]. Independent roles of these risk factors in the development of atherosclerosis are not, however, fully understood.
Dysregulated production of adipocytokines is linked to the pathogenesis of cardiovascular risk factors such as diabetes mellitus [17]. The main findings of this present study are that fasting blood glucose was independently associated with hs-CRP (β = 0.227, p < 0.05) as well as omentin-1 (β = 0.254, p < 0.01) in the study subjects. Our data indicates that obesity and inflammation are closely associated with glucose metabolism impairment [18]. It has previously been proposed that obesity is causally linked to a chronic low-grade inflammatory state, which contributes to the development of metabolic dysfunction [6,19].
It is unclear why omentin-1 and hs-CRP levels were not correlated with each other, but adipose tissue is known to secret several adipokines that have important roles in the initiation of insulin resistance or endothelial dysfunction [20,21]. Therefore, measurement of visceral adipose mass and/or levels of other adipokinesin future studies could help elucidate why these two markers did not correlate together in our study.
Levels of inflammatory cytokines (like TNF-α and IL-6) were found to be increased in obese and diabetic subjects [22]. Serum omentin-1 levels have been shown to be associated with obesity-related metabolic and vascular complications [23]. Overall, overweight and obesity were highly prevalent among the study population. In the current study, BMI was only an independent correlate of hs-CRP level (β = 0.369, p < 0.0001).
Serum omentin-1 levels were previously reported to be significantly reduced in obese compared with lean individuals in one study [24] but were similar between these groups in another study [25]. No association was observed between omentin-1 levels and measures of body fat (Table 2). It was suggest that obesity negatively regulates omentin expression [26]. Insulin resistance has been shown to be associated with pro-inflammatory states [27]. Inflammatory cytokines may contribute to the regulation of omentin-1 levels [28]. Many studies have shown that omentin-1 levels are negatively correlated with BMI, waist circumference, fasting insulin, and HOMA-IR index [24,29]. However, in the current study estimates of insulin resistance were derived using the HOMA-IR and QUICK-I, and were significantly related to serum omentin-1 values (r = 0.233, p < 0.05; r = -0.233, p < 0.05 respectively).
The Framingham risk score is a conventional means of predicting coronary risk in the general population [30]. Therefore, adipocytokines levels association with the presence and extent of coronary risk could independently predict the future risk of atherosclerotic diseases. Despite the lack of statistical difference in serum omentin-1 levels among the study population as stratified by the Framingham coronary risk score, there seems to be a tendency for a fall in serum omentin-1 concentrations with increasing coronary risk (Figure 1a). Several biomarkers, such as hs-CRP, have been shown to enhance Framingham risk score algorithms and were associated with increased cardiovascular risk [31]. Consequently, determination of circulating levels of novel markers like omentin-1 could have additional value in the prediction of future risk of CVD.
Conclusion
Our findings suggest that omentin-1 might be associated with the development of diabetes mellitus indirectly via insulin activity and obesity. These findings may have important implications for the pathophysiology and therapy of diabetes mellitus. However, due to the nature of the current study design, which does not allow us to infer causality between obesity and inflammation, omentin-1, longitudinal studies are warranted.
Acknowledgments
This study was supported by a grant number (KACST, 81-34) from the KACST. We would like to thank all the individuals who took part in the study. The authors have no conflict of interest. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. All authors read and approved the final manuscript.
References
-
Das UN (2001) Is obesity an inflammatory condition? Nutrition 17: 953-966.
-
Rizzo M, Rini G, Berneis K (2008) Inflammation and Atherosclerosis: Recent Insights and Future Perspectives. Antiinflamm Antiallergy Agents Med Chem 7: 150-151.
-
Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115: 1111-1119.
-
Festa A, D'Agostino R Jr, Howard G, Mykkanen L, Tracy RP, et al. (2000) Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102: 42-47.
-
Rocha VZ, Libby P (2009) Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 6: 399-409.
-
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116: 1793-1801.
-
Lago F, Dieguez C, Gomez-Reino J, Gualillo O (2007) Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3: 716-724.
-
Zhang H, Cui J, Zhang C (2010) Emerging role of adipokines as mediators in atherosclerosis. World J Cardiol 2: 370-376.
-
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, et al. (2006) Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab 290: E1253-1261.
-
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143-3421.
-
(1995) Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 854: 1-452.
-
World Health Organization. Waist Circumference and Waist-Hip Ratio. Report of a WHO Expert Consultation. GENEVA, 2008.
-
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
-
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, et al. (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85: 2402-2410.
-
Taubert K, Alexander RW, Anderson JL, Tracy RP, Vinicor F, et al. (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107: 499-511.
-
Casas JP, Shah T, Hingorani AD, Danesh J, Pepys MB (2008) C-reactive protein and coronary heart disease: a critical review. J Intern Med 264: 295-314.
-
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11: 85-97.
-
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98-107.
-
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860-867.
-
Fantuzzi G, Mazzone T (2007) Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol 27: 996-1003.
-
DeFronzo RA (2010) Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53: 1270-1287.
-
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745-751.
-
Liu R, Wang X, Bu P (2011) Omentin-1 is associated with carotid atherosclerosis in patients with metabolic syndrome. Diabetes Res Clin Pract 93: 21-25.
-
de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, et al. (2007) Omentin plasma levels and gene expression are decreased in obesity. Diabetes 56: 1655-1661.
-
Derosa G, Fogari E, D'Angelo A, Bianchi L, Bonaventura A, et al. (2013) Adipocytokine levels in obese and non-obese subjects: an observational study. Inflammation 36: 914-920.
-
Moreno-Navarrete JM, Catalan V, Ortega F, Gomez-Ambrosi J, Ricart W, et al. (2010) Circulating omentin concentration increases after weight loss. Nutr Metab (Lond) 7: 27.
-
Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365: 1415-1428.
-
Ryan AS, Nicklas BJ (2004) Reductions in plasma cytokine levels with weight loss improve insulin sensitivity in overweight and obese postmenopausal women. Diabetes Care 27: 1699-1705.
-
Pan HY, Guo L, Li Q (2010) Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract 88: 29-33.
-
Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, et al. (1998) Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation 97: 1876-1887.
-
Ridker PM (2007) C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 49: 2129-2138.





