International Journal of Neurology and Neurotherapy
Randomised Clinical Trial Comparing the Efficacy of A Gluten-Free Diet Versus A Regular Diet in A Series of Relapsing-Remitting Multiple Sclerosis Patients
Rodrigo L1*, Hernandez-Lahoz C2, Fuentes D1, Mauri G2, Alvarez N1, Vega J2 and Gonzalez S3
1Gastroenterology, University Hospital Central of Asturias, University of Oviedo, Spain
2Neurology, University Hospital Central of Asturias, University of Oviedo, Spain
3Immunology Services, University Hospital Central of Asturias, University of Oviedo, Spain
*Corresponding author:
Luis Rodrigo, Gastroenterology Service, University Hospital Central of Asturias, c/ Celestino Villamil s.n, University of Oviedo, 33.006, Spain, Tel: 00-34-985-10-80-58, Fax: 00-34-985-10-81-15, E-mail: lrodrigosaez@gmail.com
Int J Neurol Neurother, IJNN-1-012, (Volume 1, Issue 1), Review Article; ISSN: 2378-3001
Received: October 28, 2014 | Accepted: December 03, 2014 | Published: December 08, 2014
Citation: Rodrigo L, Hernandez-Lahoz C, Fuentes D, Mauri G, Alvarez N, et al. (2014) Randomised Clinical Trial Comparing the Efficacy of A Gluten-Free Diet Versus A Regular Diet in A Series of Relapsing-Remitting Multiple Sclerosis Patients. Int J Neurol Neurother 1:012. 10.23937/2378-3001/1/1/1012
Copyright: ©2014 Rodrigo L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To analyse the clinical efficacy of a Gluten-Free Diet (GFD) compared with a Regular Diet (RD) in Relapsing-Remitting Multiple Sclerosis (RRMS) patients.
Methods: Seventy-two RRMS patients were included into a prospective study. Annual relapse rate (ARR), Expanded Disability Status Scale (EDSS) and lesional activity were compared. Patients were randomly separated according to diet: (GFD, n=36) and (RD, n=36). Follow up study period was 5.3 ± 1.6 years (median 4.5 years).
Results: At the end of the study period, a clear improvement in the EDSS was observed in GFD (1.5 ± 1.4) compared with RD (2.1 ± 1.5) (p=0.001), and lesional activity (MRI) was found in 10 (28%) in GFD, compared to 24 (67%) in RD (p=0.001) [OR: 5.200; (CI-95%: 1.901- 14.220)]. Average ARR was lower in GFD (0.4 ± 0.6) compared to RD (0.6 ± 0.6) (NS).
Conclusions: A GFD has shown a neuro-protective effect in our RRMS patients.
Keywords
Randomised Clinical trial, Gluten-free diet, Regular diet, Relapsing-remitting, Multiple Sclerosis
Introduction
Multiple sclerosis (MS) is a chronic disease of unknown aetiology that is associated with autoimmunity and characterized by the presence of disseminated demyelinating lesions in the Central Nervous System (CNS). Activated, potentially autoimmune T cells cross the blood-brain barrier and produce inflammatory plaques and axonal loss in the brain, spinal cord or optic nerves. The result is the accumulation of demyelination and gliosis areas in the CNS. MS affects about 0.1% of the population worldwide. The relapsing-remitting form of MS (RRMS) is the initial course of more than 80% of individuals with MS. The diagnosis of MS requires that the symptoms and signs of CNS white matter involvement are disseminated temporally and spatially, with supporting evidence from MRI findings and the presence of oligoclonal bands of immunoglobulin G in the cerebrospinal fluid (CSF) if needed [1,2].
Coeliac disease (CD) is a common condition that affects 1-2% of the population worldwide and is more prevalent in females than males by a ratio of 2:1. The atypical form, which is more frequent in adult individuals, refers to CD that presents not with prominent gastrointestinal symptoms but with extra-intestinal manifestations [3-5].
Autoimmune disorders (AIDs) occur approximately 10 times more frequently in CD patients than in the general population. The most predominant associated diseases are autoimmune thyroiditis, type 1 diabetes mellitus (T1DM), Sjögren's syndrome (SS), Addison's disease (AD), autoimmune type 1 hepatitis (AIH), psoriasis and biliary cirrhosis primary (CBP), among others [6-12]. The association of AIDs with CD is considered to be mainly owing to a shared genetic tendency. CD is approximately 10 times more common than MS. When both diseases occur in a patient, CD is frequently silent, and the patient is initially diagnosed with an AID. In CD, only a strict gluten-free diet (GFD) remains the mainstay for a safe and effective treatment. Several AIDs may also improve upon patients observing a GFD because neurological syndromes are associated with gluten sensitivity in patients with and without evidence of CD [13-17]. According to this association between MS and CD, we studied the influence of a GFD on a sample of MS patients who voluntarily agreed to follow it for a prolonged period of time to assess the changes observed compared with a control group on a "regular" diet.
Methods
Patients
We conducted a prospective, controlled study of a consecutive series of patients diagnosed with RRMS who previously attended a specialized review of demyelinating diseases at the Neurology Service, University Hospital Central of Asturias (a tertiary level reference centre that serves an area of about 250,000 people), located in the Northern Spain, over the period of one year (January to December 2008).
A total of 105 patients with a previous diagnosis of RRMS were invited to voluntarily participate in a study to detect a possible clinical improvement with a GFD. The only exclusion criterion was MS patients with primary or secondary progressive forms (PP or SP). RRMS patients who agreed to participate voluntarily were referred for clinical consultation in the Hospital's Gastroenterology Department, where they were evaluated by a gastroenterologist (LR) who specializes in the study of intestinal diseases of the small bowel.
This evaluation included a specific clinical history and a number of analytical determinations that carried out systematically on all patients. Before inclusion in the study, patients gave written informed consent, and the study was previously approved by the Ethics and Research Committee of our Hospital in compliance with the modified Helsinki Declaration Recommendations.
All of the patients were previously diagnosed with RRMS at the Demyelinating Diseases Office. They returned regularly every six months for outpatient examinations and revisions and were monitored by the same neurologist (CHL).
MS studies
The neurological diagnosis was established according to Mac Donald's criteria (Polman-2005 revision) [18]. All patients were observed to have lesional temporo-spatial dissemination, as assessed by the patient's clinical history of relapses, neurological examination, and the presence of gadolinium-enhanced lesions in the brain and/or spine in Magnetic Resonance Imaging (MRI). Cerebrospinal fluid (CSF) analysis for the presence of oligoclonal bands (OCB) and the determination of visual-evoked potentials (VEP) and somatosensory potentials (SSP) were performed in most of the patients.
At the time of inclusion, the average annual rate of relapses was assessed as the rate of activity in the previous year. The Expanded Disability Status Scale (EDSS) [19] was included as the degree of physical disability that existed at a given time.
MRI evaluation
At the baseline period, all patients underwent a brain and spinal cord MRI in a Sigma HD 1.5T MR Imaging Scanner (General Electric, Milwaukee, Wisconsin, US) to assess the presence of demyelinating lesions before and after the administration of gadolinium (15ml IV Gadovist™ 1.0mmol/ml). The MRI scan was repeated once each year until the conclusion of the study, and usually using included medium contrast agent. Lesional activity was defined as positive when the number of T2-weighted lesions increased or new contrast-enhanced T1-weighted lesions appeared on the MRI scan at the conclusion time.
Laboratory tests
At each visit, the patients underwent a complete cell blood count in an autoanalyser with an automatic cell counter, model R Cell-DYN 3500 (Abbott Lab), and a thorough study of coagulation with an ACL type autoanalyser 3000 (Lab. Menarini). Iron deficiency anaemia was defined as a haemoglobin level below 12g/dl in both sexes.
We also measured a broad analytical biochemical panel, including the following parameters: an iron metabolism study, including serum iron levels (60-140mcg/ml), Transferrin saturation index (TSI) (25-45%) and serum ferritin levels (13-150 ng/ml); Liver function tests (LFTs), including the serum levels of Alkaline phosphatase (AP; 70-120 U/l, Aspartate transaminase (AST; 1-31U/l), Alanine transaminase (ALT; 1-31U/l), Gamma-glutamyl-transpeptidase (GGT; 25-50U/l) and serum bilirubin; measurement of total serum calcium, folic acid, vitamin B-12 and creatinine; total cholesterol (150-240mg/dl) and HDL and LDL fractions and triglycerides; and urea, glucose, total protein and albumin and acute phase reactants such as CRP (C-Reactive Protein). Serum immunoglobulins (IgG, IgA, IgM) were also quantified by nephelometric techniques. The circulating levels of Thyroid Stimulating Hormone (TSH) (normal range, 0.25-5.0mU/l) with serum levels of thyroid hormones (T3 and T4) were measured, and a systematic analysis of urine with sediment was performed. All measurements were performed using a modular Automatic Autoanalyser, Hitachi model SXA-PPBD (Roche) using enzymatic or kinetic procedures.
When LFTs were persistently altered, the anti-mitochondrial antibody (AMA) levels were measured by an Indirect Immuno-Fluorescence technique (IFI) on Hep-cell line 20-10 (Euro-Immun, Lübeck, Germany).
CD Studies
Serological markers
For CD screening, the quantification of Anti-tissue Transglutaminase type 2 IgA (tTG-2) by commercial ELISA (Phadia Diagnostics, Uppsala, Sweden) was used as the only serological marker. This marker was considered positive for values > 2U/ml because this threshold has a higher diagnostic sensitivity [20].
Genetic markers
To study genetic susceptibility to CD, we searched for the two most commonly used markers, HLA-DQ2 (DQA1 * 0501 and DQB1 * 0201) and HLA-DQ8 (DQA1 * 0301 and DQB1 * 0302), by PCR using specific primers and a commercial kit, (HLA System ® Domino Protrans Celiac Disease, Protrans, Ketsch, Germany).
Duodenal biopsy studies
An upper gastrointestinal endoscopy with multiple (from 4-6) biopsies from the first and second portion of the duodenum was performed on all patients. The samples obtained from the small intestine were routinely stained with the standard dyes Haematoxylin and Eosin (HE), and specific monoclonal antibodies were used to identify CD3-positive intraepithelial lymphocytes (IELs) and quantify the total number of IELs per 100 epithelial cells. Duodenal biopsies were analysed by a pathologist with expertise in CD and classified into the following types, according to the pathological classification for the diagnosis of CD, which was described by Marsh in 1992 [21], and later modified in 1999 by Oberhüber et al. [22]. Stage 0, Histologically nomal duodenum; Stage 1, Increase in the total IEL count, with a population equal to or higher than 25% of all epithelial cells; Stage 2, Hyperplasia of the crypts and/or diffuse chronic inflammatory infiltrate in the lamina itself; and Stage 3, Villous atrophy presence, subdivided into 3a) Mild, 3b) Moderate and 3c) Severe. All patients were initially offered a GFD, which they agreed to continue on an on-going basis.
Randomization Criteria
Of the 105 patients who were initially invited to participate in the study, 33 (31%) refused. Consequently, 72 patients were enrolled, of whom only 36 (50%) strictily followed a GFD throughout the duration of the study (which took a median of 4.5 years), Group 1. The remaining 36 (50%), who left the GFD soon, or committed frequent irregularities during the 6 first months of the inclusion, constituted Group 2, in which a regular diet was followed and this group, was used as "controls" (Figure 1).
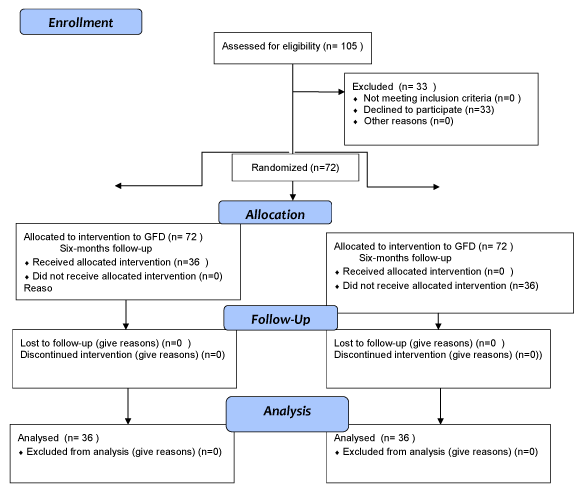
Figure 1: CONSORT 2008 flow diagram.
View Figure 1
Therefore, patients were assigned to the groups according to their degree of compliance and adherence to the GFD and were confirmed to belong to each group in consecutive reviews throughout the study period, which were conducted by examining the patient's history as reported by the patients and their families and by performing analytical tests.
Length of the study periodThe median duration of the study period was 4.5 years. A comparative study was performed between the baseline visit and the end of the study for each group and between groups.
Statistics
Continuous data are expressed as mean ± standard deviation (SD). Categorical data are presented as numbers and percentages (%). When the studied variables were not normally distributed, non-parametric statistical methods were used. For categorical variables, the Χ2 test was used. If the continuous data followed a normal distribution, a t-test was used. Differences between groups were evaluated by analysis of variance (ANOVA) followed by Fisher's test for post-hoc analysis. All statistical assessments used, were two-tailed. Statistical calculations were performed using SPSS 15.0 software (SPSS Inc., Chicago Il.) and a p value less than 0.05, was considered as statistically significant.
Results
Patients were divided according with the diet type into 2 groups: I (GFD) and II (RD). In demographic data, most of the participants were females [89% (I) vs 78% (II)]; Mean age, was very close [42 ± 11 (I) vs 44 ± 8 (II) ys]; MS age onset, was similar in both groups: [31 ± 10 (I) vs 34 ± 9 (II) ys]; MS duration was [11 ± 6 vs 10 ± 5 ys] before entrance into the study. They have received previously ß-IFN treatment in 47% (I) vs 61% (II) and have got previous pregnancies in 56% (I) vs 50% (II). No differences were found between both groups in all the parameters analyzed.
The baseline neurological records of the clinical isolated manifestations of encephalitis were [33%(I) vs 36% (II)], myelitis [67% (I) vs 64%(II)] and optical neuritis [19%(I) vs 19% (II)] and very similar. The presence of oligoclonal bands in the cerebrospinal fluid was found in [75%(I) vs 67% (II)]. The visual evoked potentials were positive in [64%(I) vs 64% (II)]. The somatosensory potentials were present in [67%(I) vs 78%(II)]. The mean baseline annual relapse rate observed was [1.0 ± 0.3 (I) vs 1.2 ± 0.4(II)], the mean basal EDSS was [1.7 ± 1.0 (I) vs 1.7 ± 1.1(II)]. The mean number of active lesions in the MRI was in percentages of [36%(I) vs 33%(II)]. Also we didn't found any differences between both groups in all the parameters analysed.
The haematological analysis showed in the haemoglobin levels (g/dl) these results: [13.2 ± 1.4 (I) vs 13.5 ± 1.3 (II)]; WBC count x103/ml. was of: [6.1 ± 2.2 (I) vs 6.9 ± 3.0 (II)], platelet count x103/ml. was of [242.6 ± 60 (I) vs 243.4 ± 47 (II)] without differences between groups. Serum iron levels in mcg/ml were [88 ± 37 (I) vs 89 ± 38 (II)], ferritin levels in ng/ml [87 ± 115 (I) vs 86 ± 91 (II)]. Total cholesterol in mg/ml, was (197 ± 36 (I) vs 196 ± 34 (II)], and liver function tests, didn't show any differences between groups.
TSH mean values expressed in U/ml were [2.3 ± 1.5 (I) vs 2.1 ± 2.0 (II)] without differences. Only the presence of anti-TPO antibodies evaluated in percentages [39 (I) vs 14 (II)] and the ANAs [25 (I) vs 6 (II)] showed differences (p< 0.05).
We found a higher prevalence of associated diseases in all MS patients, mainly of an autoimmune nature and possibly related to gluten intolerance, but the only significant difference was that iron deficiency anaemia was more common in Group 1, than in Group 2 (p< 0.005) (Table 1).
![]()
Table 1: Associated diseases and their types.
View Table 1
The results of the studies regarding the presence of serological, genetic and histological markers of gluten intolerance in the two groups are shown. We didn't found any significant difference regarding the mean tTG levels, the HLA-DQ2 and DQ8 genetic markers and the duodenal biopsy findings between both groups (Table 2).
![]()
Table 2: Gluten-related serological, genetic and histological findings.
View Table 2
We analyzed the possible influence of the HLA-DQ2 and DQ8 status, on the Annual Relapse Rate, EDSS, and MRI after the treatment between RD and GFD groups and we did not find any significant correlation. We did not determine the HLA-DRB1*1501 status, on this study.
The response to the GFD at the end of the follow-up period showed a clear improvement in the EDSS values (mean and median) and a lower lesion activity, as determined by MRI scans in Group 1, compared with Group 2 (p < 0.005) (Table 3).
![]()
Table 3: Clinical and Radiological Features at 4.5 years follow-up.
View Table 3
Discussion
The present study examined 72 patients with RRMS, 36 of whom followed a GFD for a median of 4 years. When patients on a GFD were compared with a group of equal size that followed a standard diet, we found that patients following a GFD presented a decrease in EDSS values at the end of monitoring, which translated into an improvement in their physical state and a lower lesional activity by MRI, compared with a group following a normal diet (p = 0.001 for both results). There was also a decrease in the average annual rate of relapses in Group 1 with respect to Group 2, although it was not significant (NS).
These data confirm the initial hypothesis that a GFD seems to produce an objective clinical benefit in and a clear neuroprotective effect in these patients. This is a pioneering study in that we recommended a GFD to patients regardless of the presence of concomitant CD, the existence of an underlying enteropathy or an association with intolerance to gluten.
One of the weaknesses of the study is the selection of patients. A GFD was initially offered to all patients. They were subsequently divided into two groups, and the second group comprised those patients who chose not to adhere to this type of diet or resigned at the beginning of the study (in the first six months visit). The adherence of the GFD was confirmed by specific questioning of patients and their families at each biannual visit over a median follow-up of four and a half years.
Both groups included equal numbers of patients (n=36) and showed no clinical differences in terms of age of onset and duration of RRMS. In group 1, 47% of the patients were receiving immuno-modulatory treatment with IFN-beta compared with 61% in group 2 (NS). There were no differences in history of pregnancy between the two groups. Both groups were also homogeneous with regard to the average annual rate of relapses and baseline EDSS. Neurological findings (VEPs, SEPs and the presence or absence of OCB in the CSF) were comparable between groups. There were also no differences in relation to MRI findings as an indicator of disease activity between groups.
Baseline blood counts and biochemistry, including liver and thyroid function tests, showed no different differences values. It should be mentioned that the presence of circulating anti-peroxidase from thyroid (TPO type) and anti-nuclear (ANA) antibodies was slightly higher in group 1, than in group 2 (p < 0.05 for both results).
The high frequency of various kinds of associated diseases, such as skin alterations, endocrine disorders (primarily hypothyroidism), increased liver function tests and osteoporosis (many of them probably autoimmune in nature), with no differences between groups, is remarkable. Iron deficiency anaemia was the second most frequent associated disease after dermatitis and occurred at a higher rate in group 1 (56%) than in group 2 (22%) (p = 0.004).
A high level of anti-tissue transglutaminase-2 (tTG-2) IgA in the serum of the patients is an important serological marker in the diagnosis of CD and correlates well with the severity of villous atrophy in the small intestinal biopsy. Anti-tTG-2 antibody serology may be negative in the presence of partial villous atrophy or in subjects on a GFD prior to testing. Regarding the serological markers of gluten intolerance, the only marker found was tTG, whose mean values were slightly higher in group 1 (6.3 ± 3.1 U/l) than in group 2 (1 ± 0.5 U/l) (NS), which may be related to the wide range of values used. This variability occurs most often in clinical practice, and a large international study has demonstrated a wide variability in the sensitivity (69-93%) and specificity (96-100%) of this measurement in a total of 20 laboratories [23]. There were also differences in the threshold of significance between different commercial reagents for the serological diagnosis of CD [24]. During the detection of CD in everyday clinical practice, the diagnostic value used is lower than that reported in the literature, especially in the absence of villous atrophy, which shows a good correlation with the diagnosis of CD [25]. Several studies have confirmed that the diagnostic sensitivity of tTG decreases to below 30% in patients with lymphocytic enteritis (Marsh1) [26].
Neurological dysfunction may be the only initial manifestation of gluten sensitivity. Antibodies to one isotype of tTG, namely, the sixth variety (tTG-6; both IgG and IgA), show a higher prevalence in gluten-related ataxia and can be used as sensitive and specific markers of neurological disorders associated with CD [27]. Reichelt et al. found a significant increase in the anti-gliadin IgA class in a series of 36 patients with MS, whereas they found no elevation in anti-tTG or anti-endomysium antibodies [28]. Regarding the genetic markers that are most commonly associated with gluten intolerance, such as the DQ2 and DQ8 subtypes of HLA-II, no differences were found between groups or in healthy bone marrow donors. In CD, DQ2 is the predominant genetic marker (90-95%), with DQ8 having a much lower frequency (5-10%). Individuals who are negative for both common genetic markers have a low risk, but at least, there are also about 5% of patients that are negative for both DQ2 and DQ8, as confirmed in a recent multinational European study [29].
Although HLA-DQ2 is the major genetic risk factor for CD, Romanos et al. [30] have shown that patients with CD who are carrying 13 or more non-HLA risk alleles are more at risk of CD than carriers of 0-5 alleles. This model produced an increased sensitivity of 6.2% compared with the isolated use of HLA as the unique marker.
Histological examination of the small intestine remains the gold standard for the diagnosis of CD. Biagi et al. [31] demonstrated that minimal intestinal lesions in the absence of positive serology are associated most often with CD. Deposits of IgA against tTG-2 in mucosal tissue of the small intestine suggest that the patient may be sensitive to gluten despite having a normal duodenal villous architecture [32].
In our study, we confirmed that 8 patients had lesions of mild villous atrophy (Marsh = 3) with all of the histological criteria of CD. All of these patients were in group 1, in which there was a clear predominance of coeliac patients (22.2%) compared with group 2 (0%). Our research group has recently reported an increased prevalence of CD patients (11%) among patients with RRMS, which is far greater than the 1-2% found in the general population [33].
Although the findings of a duodenal biopsy are important to confirm the diagnosis of CD, some reports have confirmed the high correlation between high serum tTG levels (> 100 U/l) and the presence of villous atrophy, suggesting that duodenal biopsies could be avoided, at least in children, in this situation [34].
MS has a multifactorial aetiology and the contribution of anti-tTG and anti-gliadin antibodies (AGA) may be interesting in this disease, not only from a diagnostic point of view but also because they may contribute to its pathogenesis. Historically, a GFD has occasionally been used in a speculative way to treat MS. There are only anecdotal descriptions of the use of a GFD, targeting a possible beneficial effect, in isolated cases with this disease [35-37], and subsequent studies found some benefit from their implementation [38].
Although there are discrepancies about the possible association of CD with demyelinating diseases, such as MS and neuromyelitis optica (NMO), the possibility is well documented, and good responses to a GFD have been observed in some cases [39-41].
Recently, authors from Israel studied the presence of antigliadin IgG in a series of 98 MS patients and found that it was present in 7 patients, compared with 140 control cases present in two (p = 0.03). They also measured the level of anti-tTG IgG and found positivity in 4 patients in group I and in none of the controls (p=0.02). The authors concluded that there is a strong association between the presence of such antibodies and MS, postulating that a GFD would be advisable, especially in patients who are positive for gluten-related circulating antibodies [42].
In the present study, we also found that a GFD has a neuroprotective role in a majority of MS patients, especially in improving the physical capacity, as determined by EDSS, and the activity of lesions seen on MRI. The higher prevalence of CD in group 1, has probably influenced these favourable results.
A high prevalence of associated CD has been detected in our RRMS patient series; thus, the GFD may produce a beneficial effect on both diseases in these cases. These preliminary findings should be confirmed, however, in larger comparative studies that are designed prospectively with a multicenter participation and a longer follow-up period.
Statement of Interests
All the authors participated in this study declare that they have no competing interests, neither private nor public, that they have received in the last two years, at least.
Author's Contributions
Conception and design by LR and CHL. Analysis and interpretation of the data by LR SG and CHL. Drafting of the article by DF, GM, NA, JV and SG. Critical revision of the paper for intellectual content by LR, SG and CHL. All authors read and approved the final manuscript.
Consort Statement
All the authors participants in this study ensure that the Consort description have been fully addressed within their submitted paper.
References
-
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372: 1502-1517.
-
Ramagopalan SV, Dobson R, Meier UC, Giovannoni G (2010) Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol 9: 727-739.
-
Green PH, Alaedini A, Sander HW, Brannagan TH 3rd, Latov N, et al. (2005) Mechanisms underlying celiac disease and its neurologic manifestations. Cell Mol Life Sci 62: 791-799.
-
Green P, Cellier C (2007) Celiac disease. N Engl J Med 357: 1731-1743.
-
Freeman HJ, Chopra A, Clandinin MT, Thomson AB (2011) Recent advances in celiac disease. World J Gastroenterol 17: 2259-2272.
-
Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, et al. (2010) Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 123: 183.
-
Talal AH, Murray JA, Goeken JA, Sivitz WI (1997) Celiac disease in an adult population with insulin-dependent diabetes mellitus: use of endomysial antibody testing. Am J Gastroenterol 92: 1280-1284.
-
Lawson A, West J, Aithal GP, Logan RF (2005) Autoimmune cholestatic liver disease in people with coeliac disease: a population-based study of their association. Aliment Pharmacol Ther 21: 401-405.
-
Iltanen S, Collin P, Korpela M, Holm K, Partanen J, et al. (1999) Celiac disease and markers of celiac disease latency in patients with primary Sjögren's syndrome. Am J Gastroenterol 94: 1042-1046.
-
O'Leary C, Walsh CH, Wieneke P, O'Regan P, Buckley B, et al. (2002) Coeliac disease and autoimmune Addison's disease: a clinical pitfall. QJM 95: 79-82.
-
Ojetti V, Aguilar Sanchez J, Guerriero C, Fossati B, Capizzi R, et al. (2003) High prevalence of celiac disease in psoriasis. Am J Gastroenterol 98: 2574-2575.
-
Dickey W, McMillan SA, Callender ME (1997) High prevalence of celiac sprue among patients with primary biliary cirrhosis. J Clin Gastroenterol 25: 328-329.
-
Pengiran Tengah CD, Lock RJ, Unsworth DJ, Wills AJ (2004) Multiple sclerosis and occult gluten sensitivity. Neurology 62: 2326-2327.
-
Frisullo G, Nociti V, Iorio R, Patanella AK, Marti A, et al. (2008) Increased expression of T-bet in circulating B cells from a patient with multiple sclerosis and celiac disease. Hum Immunol 69: 837-839.
-
Ferrò MT, Franciotta D, Riccardi T, D'Adda E, Mainardi E, et al. (2008) A case of multiple sclerosis with atypical onset associated with autoimmune hepatitis and silent coeliac disease. Neurol Sci 29: 29-31.
-
Hadjivassiliou M, Sanders DS, Grünewald RA, Woodroofe N, Boscolo S, et al. (2010) Gluten sensitivity: from gut to brain. Lancet Neurol 9: 318-330.
-
Hernandez-Lahoz C, Mauri-Capdevila G, Vega-Villar J, Rodrigo L (2011) [Neurological disorders associated with gluten sensitivity]. Rev Neurol 53: 287-300.
-
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 58: 840-846.
-
Gaspari M, Saletti D, Scandellari C, Stecchi S (2009) Refining an automatic EDSS scoring expert system for routine clinical use in multiple sclerosis. IEEE Trans Inf Technol Biomed 13: 501-511.
-
Mariné M, Fernández-Bañares F, Alsina M, Farré C, Cortijo M, et al. (2009) Impact of mass screening for gluten-sensitive enteropathy in working population. World J Gastroenterol 15: 1331-1338.
-
Marsh MN (1992) Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 102: 330-354.
-
Oberhuber G, Granditsch G, Vogelsang H (1999) The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 11: 1185-1194.
-
Li M1, Yu L, Tiberti C, Bonamico M, Taki I, et al. (2009) A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol 104: 154-163.
-
Fernández E, Riestra S, Rodrigo L, Blanco C, López-Vázquez A, et al. (2005) Comparison of six human anti-transglutaminase ELISA-tests in the diagnosis of celiac disease in the Saharawi population. World J Gastroenterol 11: 3762-3766.
-
Naiyer AJ, Hernandez L, Ciaccio EJ, Papadakis K, Manavalan JS, et al. (2009) Comparison of commercially available serologic kits for the detection of celiac disease. J Clin Gastroenterol 43: 225-232.
-
Esteve 1, Rosinach M, Fernández-Bañares F, Farré C, Salas A, et al. (2006) Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut 55: 1739-1745.
-
Hadjivassiliou 1, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, et al. (2008) Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol 64: 332-343.
-
Reichelt K1, Jensen D (2004) IgA antibodies against gliadin and gluten in multiple sclerosis. Acta Neurol Scand 110: 239-241.
-
Margaritte-Jeannin 1, Babron MC, Bourgey M, Louka AS, Clot F, et al. (2004) HLA-DQ relative risks for coeliac disease in European populations: a study of the European Genetics Cluster on Coeliac Disease. Tissue Antigens 63: 562-567.
-
Romanos , van Diemen CC, Nolte IM, Trynka G, Zhernakova A, et al. (2009) Analysis of HLA and non-HLA alleles can identify individuals at high risk for celiac disease. Gastroenterology 137: 834-840, 840.
-
Biagi F, Bianchi PI, Campanella J, Badulli C, Martinetti M, et al. (2008) The prevalence and the causes of minimal intestinal lesions in patients complaining of symptoms suggestive of enteropathy: a follow-up study. J Clin Pathol 61: 1116-1118.
-
Koskinen O, Collin P, Karponay-Szabo I, et al. (2008) Gluten-dependent small bowel mucosal transglutaminase 2-specific IgA deposits in overt and mild enteropathy coeliac disease. J Pediatr Gastroenterol Nutr 47: 436-442.
-
Rodrigo L, Hernández-Lahoz C, Fuentes D, Alvarez N, López-Vázquez A, et al. (2011) Prevalence of celiac disease in multiple sclerosis. BMC Neurol 11: 31.
-
Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, et al. (2009) Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol 15: 4775-4780.
-
Mac Dougall R (1973) No bed of roses. World Med 8: 98-99.
-
Matheson NA (1974) Letter: Multiple sclerosis and diet. Lancet 2: 831.
-
Nicoletti A, Patti F, Lo Fermo S, Sciacca A, Laisa P, et al. (2008) Frequency of celiac disease is not increased among multiple sclerosis patients. Mult Scler 14: 698-700.
-
Hernández-Lahoz C, Rodríguez S, Tuñón A, Saiz A, Santamarta E, et al. (2009) [Sustained clinical remission in a patient with remittent-recurrent multiple sclerosis and celiac disease gluten-free diet for 6 years]. Neurologia 24: 213-215.
-
Jacob S, Zarei M, Kenton A, Allroggen H (2005) Gluten sensitivity and neuromyelitis optica: two case reports. J Neurol Neurosurg Psychiatry 76: 1028-1030.
-
Jarius S, Jacob S, Waters P, Jacob A, Littleton E, et al. (2008) Neuromyelitis optica in patients with gluten sensitivity associated with antibodies to aquaporin-4. J Neurol Neurosurg Psychiatry 79: 1084.
-
Bergamaschi R, Jarius S, Robotti M, Pichiecchio A, Wildemann B, et al. (2009) Two cases of benign neuromyelitis optica in patients with celiac disease. J Neurol 256: 2097-2099.
-
Shor DB1, Barzilai O, Ram M, Izhaky D, Porat-Katz BS, et al. (2009) Gluten sensitivity in multiple sclerosis: experimental myth or clinical truth? Ann N Y Acad Sci 1173: 343-349.





