Malignant melanomas of the nasal mucosa represent approximately 1% of all melanomas and reported examples often arise in areas of pigmentation of the nasal mucosa. Melanin pigmentation has been reported to occur in the nasal glands and dendritic cells but melanomas arising from areas of pigmented (melanocyte containing) nasal glands have not been reported to our knowledge.
A primary sinonasal malignant melanoma arising in association with melanosis of the nasal mucosa and seromucinous glands is reported in a seventy-six-year-old Caucasian male. Focal pigmentation of the seromucinous glands and surface epithelium of the nasal septum was present and associated with immunoreactivity for HMB-45 and melan-A and a positive Fontana-Masson reaction. A malignant melanoma was present, arising within the area of the melanosis.
The present case demonstrates that nasal melanomas may arise from areas of nasal seromucinous glands containing melanocytes and melan pigment.
Nose, Nasal glands, Melanoma, Pigment, Melanin
Pigmentary disorders of the nasal mucosa and submucosal glands are rare and include melanocytic oncocytic metaplasia [1,2], melanocytic hyperplasia of the nasal respiratory epithelium, and primary malignant melanoma [3]. Intranasal malignant melanoma represents approximately 1% of total malignant melanomas arising at all body sites [4]. Dendritic melanocytes have been identified within the intranasal respiratory epithelium and may represent the site of origin for many if not most nasal malignant melanomas [4-6].
Melanotic oncocytic metaplasia shows coexistence of oncocytic metaplasia and melanin pigmentation within the same gland [1,7,8]. The precise origin of the melanin pigment within the oncocytic epithelium is unclear. Melanocytes are known to exist within the stroma and epithelium of the nasal cavity and paranasal sinuses [5,6,9] and these cells may be the source of the melanin pigment within the oncocytic epithelium [7]. Cases of melanotic oncocytic metaplasia demonstrate dendritic melanocytes which are S-100 positive but HMB-45 negative. To date melanotic oncocytic metaplasia has not been associated with the development of malignant melanoma [1,2,10,11]. In this review, we report a case of melanosis of nasal seromucinous glands arising within a Caucasian male and associated with a malignant melanoma primary within the nasal cavity. In our case, the seromucinous glands did not show oncocytic metaplasia, but melanin was present in a pattern similar to that reported in melanotic oncocytic metaplasia.
This report was approved by the Veteran's Administration Institutional Review Board.
A 75-year-old man presented with a one month history of left ear pain, drainage and decreased hearing in the left ear. In addition, he reported chronic left nasal congestion with a constant inability to breathe through his left nostril. He was recently treated for acute sinusitis at an outside hospital which resolved after antibiotic therapy, however, his ear symptoms and nasal congestion persisted. The patient denied epistaxis, but did report small amounts of hemoptysis daily.
A flexible nasolaryngoscopy was performed which demonstrated a small mass extending off the posterior edge of the left septum with associated dark mucosa (Figure 1) extending beyond the mass up to the level of the superior turbinate to the left nasal floor and on the right around the choana to the right nasal floor. In addition, there was significant left nasal septal deviation. A biopsy of the nasal mass demonstrated a spindle cell melanoma. A posterior septectomy was performed including bilateral superior and right middle turbinectomy in addition to excision of the sphenoid face and bilateral nasal floor mucosa.
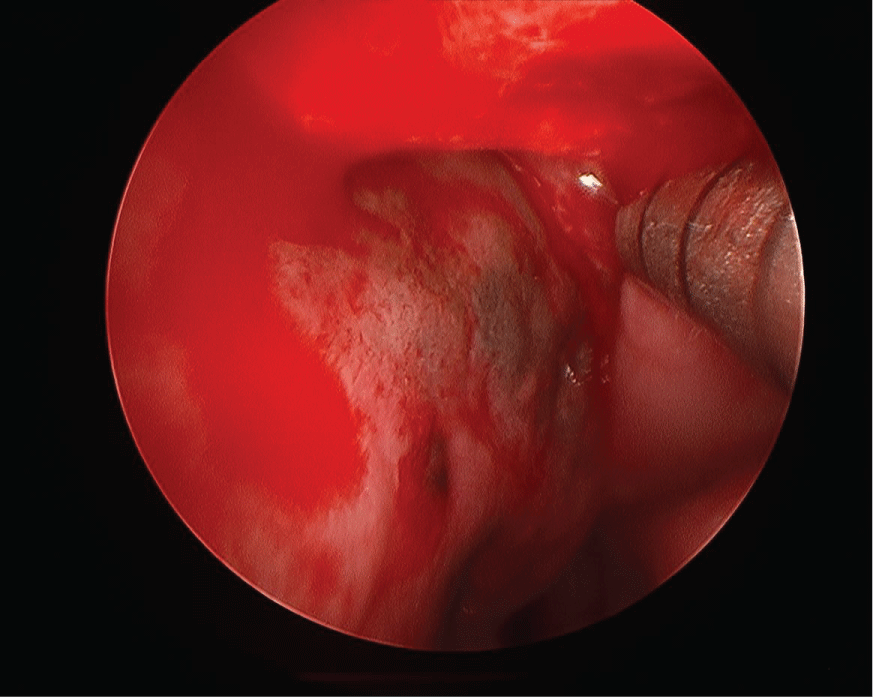 Figure 1: Flexible nasolaryngoscope image of pigmented area and mass at posterior edge of nasal septum. View Figure 1
Figure 1: Flexible nasolaryngoscope image of pigmented area and mass at posterior edge of nasal septum. View Figure 1
The resection specimen was composed of a 3.7 × 3.5 × 0.5 cm aggregate of soft tissue, bone and cartilage containing areas of blue-brown discoloration of the mucosal surface.
Histologic evaluation disclosed a pigmented focally ulcerated mass surrounded by a zone of pigmentation within both the mucosa and some of the seromucinous glands. The mass was composed of atypical oval to polygonal cells with scant to modest amounts of cytoplasm containing scattered brown pigment. Morphologically, the neoplasm was consistent with malignant melanoma (Figure 2A and Figure 2B). No in-situ component of the melanoma was seen. The mucosa over the mass was ulcerated and the neoplastic cells invaded the underlying soft tissues and surrounded and destroyed the entrapped seromucinous glands. At the periphery of the mass, the seromucinous glands contained pigment morphologically identical to that seen in the melanoma (Figure 3A). The pigment lay in the cuboidal to low column cells lining the lumen of the glands (Figure 3B). Immunohistochemical staining for S-100 protein, HMB-45 and melan A showed the glandular cells to be immunoreactive for these three antigens (Figure 4A, Figure 4B and Figure 4C). The cuboidal cells also contained granular pigment positive by the Fontana-Masson reaction. This black pigment was most intense near the laminal surface of the cells (Figure 5). Occasional Fontana-Masson positive cells were present in the overlying mucosa near the melanoma and occasionally loose in the stroma (Figure 6).
 Figure 2: A) Photomicrograph of mass lesion composed of atypical oral and polygonal cells representing malignant melanoma (H+E, X200); B) The oval and polygonal cells demonstrate nuclear hyperchromasia and membrane irregularities (H+E, X400). View Figure 2
Figure 2: A) Photomicrograph of mass lesion composed of atypical oral and polygonal cells representing malignant melanoma (H+E, X200); B) The oval and polygonal cells demonstrate nuclear hyperchromasia and membrane irregularities (H+E, X400). View Figure 2
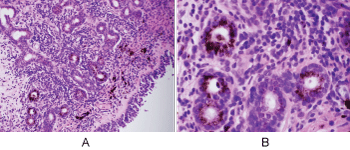 Figure 3: A) Seromucinous glands of nasal mucosa containing melanin pigment (H+E, X200); B) Prominent melanosis of epithelium of glands (H+E, X600). View Figure 3
Figure 3: A) Seromucinous glands of nasal mucosa containing melanin pigment (H+E, X200); B) Prominent melanosis of epithelium of glands (H+E, X600). View Figure 3
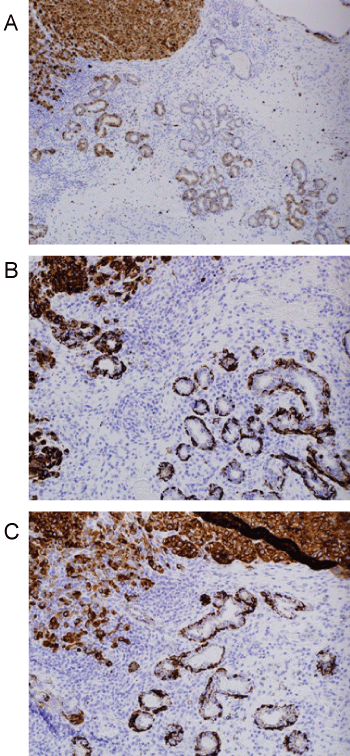 Figure 4: A) S100 stain demonstrating positivity in melanoma and adjacent glands (Immunohistochemistry, X100); B) HMB45 staining in both glandular epithelium and adjacent melanoma (Immunohistochemistry, X200); C) Reactivity for melan A in both melanoma and glands (Immunohistochemistry, X200). View Figure 4
Figure 4: A) S100 stain demonstrating positivity in melanoma and adjacent glands (Immunohistochemistry, X100); B) HMB45 staining in both glandular epithelium and adjacent melanoma (Immunohistochemistry, X200); C) Reactivity for melan A in both melanoma and glands (Immunohistochemistry, X200). View Figure 4
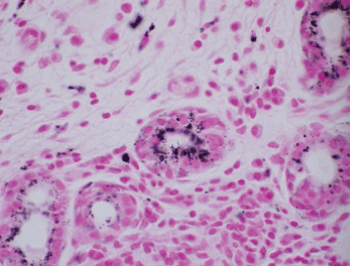 Figure 5: Fontana-Masson staining of glandular epithelium (Fontana-Masson, X600). View Figure 5
Figure 5: Fontana-Masson staining of glandular epithelium (Fontana-Masson, X600). View Figure 5
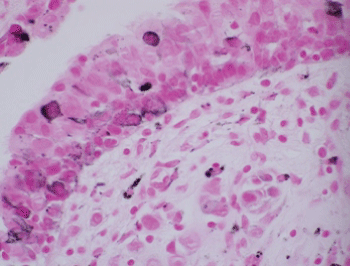 Figure 6: Focal positivity for Fontana-Masson reaction in respiratory epithelium (Fontana-Masson, X600). View Figure 6
Figure 6: Focal positivity for Fontana-Masson reaction in respiratory epithelium (Fontana-Masson, X600). View Figure 6
Mucosal melanomas are rare and are reported to have a more aggressive behavior than their cutaneous counterparts [12-17]. Sinonasal melanomas appear to be the most common of the head and neck mucosal melanomas [12,18]. Sinonasal melanomas arise in a variety of sites with approximately 55% arising in the nasal cavity, 12% to 44% in the lateral wall, 8% to 20% in the nasal septum and 10% to 50% in a single sinus [15,19,20]. Melanomas may take a variety of cell shapes with epithelioid, plasmacytoid, undifferentiated, spindle cell, clear cell and mixed types being reported [15,17,21]. The mixed type is most common followed by the epithelial type [15]. Sinonasal melanomas frequently express S-100 protein, HMB-45 and melan A [15,17]. The melanoma cells in our case were predominately oval to polygonal in shape (epithelioid) and were S-100 protein, HMB-45 and melan A reactive.
The mutational profile of mucosal melanomas including sinonasal melanomas differs from cutaneous melanomas. Unlike cutaneous melanoma, sinonasal melanomas tend to be BRAF mutation negative but show mutations in KIT [16,17,20].
Sinonasal melanomas are believed to arise from neural crest cells which migrate to the nasal mucosa [17]. Zak and Lawson demonstrated melanocytes within the nasal mucosa, glands and stroma [6]. Melanocytes within mucous glands may participate in a widespread junctional change associated with melanoma [14]. The precise site of origin for sinonasal melanomas is not clear. Several reports have disclosed pigmentary abnormalities in the mucosa near sinonasal melanomas [4,5,12,14]. The nature of these pigment abnormalities has varied among patients with some representing intramucosal dendritic melanocytes [5], others are examples of pigment incontinence in stroma [12] and still others appear to be associated with pigment deposition in sinonasal glands [14].
The pigmented areas discrete from the malignant melanoma in our case were associated with pigmented cells in the seromucinous glands. Most prior studies have not described pigmented cells in the seromucous glands as seen in our case [15-21]. The luminal lining cells contained not only melanin pigment (positive Fontana-Masson reaction) but also were immunoreactive for HMB-45, S-100 protein and melan A suggesting the production of melanin in these cells. Earlier reports of melanotic oncocytic metaplasia demonstrated melanin pigment in the seromucinous glands but the cells were HMB-45 negative [1]. This finding suggested that the melanin pigment may only have been phagocytized by the oncocytic epithelium rather than produced in these cells. The precise site of origin for the melanoma in our patient is unclear because of the destructive nature of the neoplasm. However, the melanoma may have arisen within the mucosa or the seromucinous glands. Our case differs in several ways from prior reports of melanocytic oncocytic metaplasia. While two prior reports and our case had melan pigment within glandular epithelum, our case was positive for HMB-45 and melan A while the others were not. Additionally, the prior reports of melanotic oncocytic metaplasia occurred in Asian men [1,2] our patient was a Caucasian man. Finally, the seromucinous glands in our case did not show oncocytic change.
Our case confirms previous reports in that malignant melanoma may arise primarily within the nasal mucosa and that some of these melanomas may be associated with mucosal or sinonasal gland pigmentary disorders. Our case supports the suggestion by McClennan and Shivas that mucous or sinonasal glands can take part in a widespread activation of junctional melanocytes associated with melanoma [14]. As in prior cases, nasal melanoma may present as epistaxis or nasal obstruction. The malignancy risk for nasal pigmentation remains unknown and will require further studies to establish risk statistics.