International Journal of Neurology and Neurotherapy
Does the Benefit of Therapeutic Hypothermia in Refractory Status Epilepticus Depend on Seizure Etiology?
Jessica R. Fesler, Ryan Hakimi*, Emmaculate M. Fields and Andrea S. Hakimi
Department of Neurology, University of Oklahoma Health Sciences Center Oklahoma City, Oklahoma, USA
*Corresponding author:
Ryan Hakimi, Assistant Professor, University of Oklahoma Health Sciences Center, Director of Critical Care Neurology, Medical Director of Oklahoma University Medical Center Neurosciences Intensive Care Unit, 920 Stanton L. Young Blvd, Suite 2040, Oklahoma City, OK 73104, USA, Tel: 405-271-4113, E-mail: Ryan-Hakimi@ouhsc.edu
Int J Neurol Neurother, IJNN-2-018, (Volume 2, Issue 1), Original Article; ISSN: 2378-3001
Received: January 02, 2015 | Accepted: January 16, 2015 | Published: January 19, 2015
Citation: Fesler JR, Hakimi R, Fields EM, Hakimi AS (2015) Does the Benefit of Therapeutic Hypothermia in Refractory Status Epilepticus Depend on Seizure Etiology?. Int J Neurol Neurother 2:018. 10.23937/2378-3001/2/1/1018
Copyright: ©2015 Fesler JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Refractory status epilepticus (RSE) is seizure activity that persists despite acute administration of standard anticonvulsant therapy. It often occurs after cardiac arrest, indicating a poor prognosis. Therapeutic hypothermia (TH) reduces neurological injury in this patient population, and post cardiac arrest protocols now incorporate TH into the treatment algorithm. However, TH is not routinely used in RSE from other etiologies and little is known about the appropriate role of TH as an adjunct therapy.
Methods: Five consecutive patients with RSE admitted to our neurocritical care unit treated with TH were retrospectively reviewed. Three patients had anoxic brain injury post cardiac arrest and two had epilepsy secondary to remote traumatic brain injury. All five patients received similar medical treatment for RSE.
Results: The two post-traumatic epilepsy patients became seizure free with TH and remained seizure free after rewarming. Both had good neurological recovery and were discharged from rehabilitation back to baseline. Seizures persisted despite TH in the three post-cardiac arrest patients and all three expired.
Conclusion: Currently, there are only five reported cases of TH use in adult patients with status epilepticus. Our additional five cases suggest that RSE from etiologies other than anoxic brain injury post-cardiac arrest may have better outcomes with TH. The benefit of TH in RSE may depend on seizure etiology and may have a more favorable outcome in post-traumatic epilepsy patients.
Keywords
Epilepsy, Refractory status epilepticus, Therapeutic hypothermia, Cardiac arrest, Traumatic brain injury
Introduction
Hypothermia has neuroprotective properties in many acute neurological processes including anoxic brain injury, ischemic stroke, and traumatic brain injury [1-4]. It has also been shown to terminate epileptiform discharges in both human and animal models [5-7]. Given these results, there has been increasing interest in the use therapeutic hypothermia (TH) as an adjunct treatment for seizures not responsive to traditional abortive methods, i.e. refractory status epilepticus (RSE). TH reduces neurological injury from diffuse anoxia and is incorporated into many post cardiac-arrest protocols, but is not routinely used in RSE from other etiologies [3]. In current clinical practice, TH is a last resort in the most refractory cases [8]. As our experience and knowledge increases, TH may play an earlier and more integral role in protocols for treatment of status epilepticus. At minimum, it is a promising adjunct therapy in some patients, but exactly who those patients are is yet to be determined.
Methods
After Institutional Review Board approval, patient records were retrospectively reviewed for all Neurosciences Intensive Care Unit (NSICU) patients who underwent TH with RSE between January 1, 2011 and December 31, 2013.
In our facility, patients with recurrent or continuous clinical or electrographic seizures are admitted to the NSICU for continuous video electroencephalogram (cEEG) monitoring. Status epilepticus is treated with intravenous lorazepam 0.05mg/kg, which is repeated if seizures persist for two minutes after initial dose. Intravenous fosphenytoin 20mg/kg phenytoin equivalents (PE) is administered, followed by an additional 10mg/kg PE if seizures persist. After the second load of fosphenytoin, 3000 mg of intravenous levetiracetam is administered. The next step in our algorithm should seizures persist is to intubate the patient for airway protection and anticipated infusion of benzodiazepines, barbiturates, or general anesthetics while mechanically ventilated. Levetiracetam and fosphenytoin are continued as maintenance therapy. There is some variability depending on clinical circumstances, and subsequent treatment with additional anticonvulsant therapy is at the discretion of the neurocritical care attending physician.
TH is considered for patients with RSE unabated with high-dose benzodiazepine and/or barbiturate infusions or recurrent seizure with weaning of these medications. Patients are not candidates for TH if pregnant, hemodynamically unstable despite vasopressor support, body temperature less than 33 degrees Celsius prior to initiation, or the patient or patient's family have expressed wishes for limited care. TH is initiated using a surface cooling device (Artic Sun Temperature Management System, Medivance, Louisville, CO) or endovascular cooling with a central venous catheter (CoolLine, ZOLL Medical Corp., Chelmsford, MA) to achieve target temperature of 33 degrees Celsius documentation by nursing of the time target temperature is achieved. During the induction phase, if shivering is observed, the patient is started on fentanyl, midazolam, with or without cisatracurium infusion titrated to control shivering. If initiated, cisatracurium dose is titrated based on an hourly Train-Of-Four with goal 1 of 4. Sedation is titrated using bispectral monitoring index (BIS monitor, Aspect Medical Systems, Newton, MA) to goal of 40 to 60.
If seizures are abated and the patient has been at target temperature for 24 hours, continuous infusions are weaned. Thereafter, the patient is re-warmed at a rate of 0.25 degrees Celsius every hour to 36.5 degrees Celsius while is ongoing. Nursing staff records the time patient reaches 36.5 degrees Celsius. During induction, maintenance, and rewarming phases of our hypothermia protocol, complete blood cell counts, electrolytes, arterial blood gases, and coagulation studies are monitored routinely. Hemodynamic stability is maintained by vasopressors if needed. To minimize risk of deep vein thrombosis, sequential compression devices are routinely worn and patients are screened weekly with ultrasound examination of all extremities.
Case Reports
Patient 1
A 20-year-old man with a traumatic brain injury secondary to an automobile versus pedestrian accident five years prior to admission who sustained a traumatic brain injury with residual right hemiparesis and had an isolated likely secondarily generalized seizure two years prior to admission with possible right head deviation at onset was admitted to the NSICU for clinical status epilepticus which again was reported to have started with right head deviation. Neuroimaging was unchanged. Clinical generalized seizure activity was unabated by lorazepam, fosphenytoin, levetiracetam, propofol, lacosamide, and topiramate. Despite several doses of phenobarbital and increasing midazolam infusion over a ten-hour period, the patient continued to clinically seize and TH was initiated. Target temperature was maintained for approximately 42 hours and no clinical or electrographic seizure activity occurred with weaning of infusions or rewarming. Patient was transitioned to oral levetiracetam and lacosamide. Hospital course was complicated by ventilator-associated pneumonia and ileus, both of which resolved with treatment. He was discharged to inpatient rehabilitation on day 18 near pre-admission neurological and functional status.
Patient 2
A 38-year-old man with traumatic brain injury six years prior to admission with resultant baseline aphasia, subtle hemiparesis, thalamic pain syndrome, and focal epilepsy presented in status epilepticus. Prior to this event, he had not had any breakthrough seizures while on maintenance carbamazepine 400 mg twice daily since his initial injury. On admission, CSF studies were negative and neuroimaging was unchanged (Figure 1). Clinical seizures, which were reported to have started with right hemibody shaking before quick generalization, were aborted with midazolam and levetiracetam at an outside hospital. His clinical generalized seizure activity then recurred after transfer to our facility and persisted despite midazolam, fosphenytoin, levetiracetam, and lacosamide. Topiramate was added but discontinued because of resultant metabolic acidosis. Electrographic seizures continued despite propofol infusion to 200 microgram per kilogram per minute with additional periodic boluses. Propofol was stopped due to significant lipemia, and he was then placed on pentobarbital with goal of burst suppression, eventually requiring titration to 5 milligram per kilogram per hour. This was later transitioned to phenobarbital and midazolam infusions. Weaning of these infusions resulted in recurrent seizure (Figure 2).
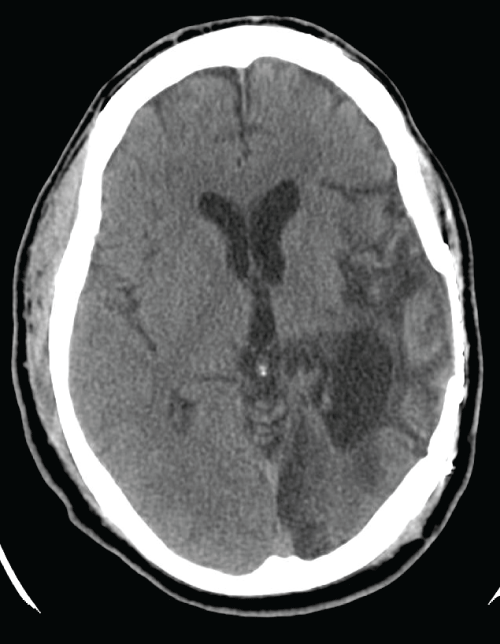
Figure 1: CT head without contrast in Patient 2 showing right fronto-parietal
encephalomalacia from remote traumatic brain injury
View Figure 1
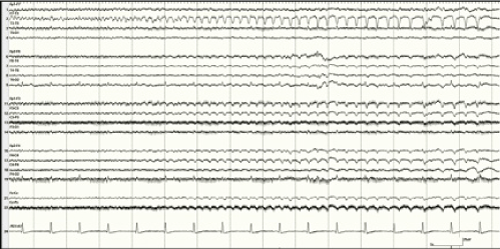
Figure 2:EEG sample in Patient 2 showing typical seizure activity prior to
initiation of TH
View Figure 2
Thus, TH was initiated on the sixth day after admission. Thereafter, continuous infusions were successfully weaned. Target temperature was maintained for 79 hours. After re-warming, he had no further electrographic or clinical seizures (Figure 3). Patient had a slow recovery from coma necessitating tracheostomy and gastrostomy tube placement, and his clinical course was complicated by pneumonia with pleural effusion and lower extremity deep vein thrombosis. He was discharged to a long term acute care facility on day 23. His mental status and communication were near pre-admission baseline with generalized deconditioning from prolonged hospital stay. Six weeks later, after reversal of his tracheostomy and gastrostomy tubes, he was transferred to inpatient rehabilitation for one month. He returned to the neurology clinic for follow up three months after discharge from NSICU at his pre-admission baseline.
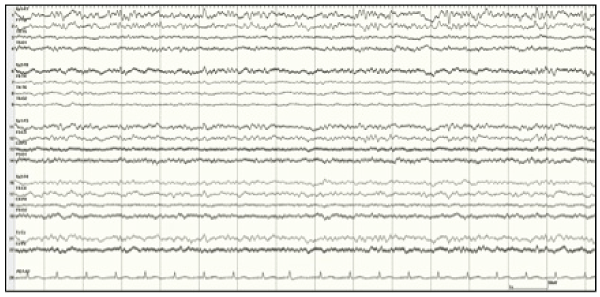
Figure 3: EEG sample in Patient 2 showing resolution of seizures after initiation of TH
View Figure 3
Patient 3
A 47-year-old woman with asthma experienced cardiac arrest due to respiratory distress and was subsequently admitted to the NSICU for TH. Initial workup and neuroimaging did not reveal an etiology for her coma. As she was cooled to target temperature, she had myoclonic jerks, was placed on cEEG, and was found to be in myoclonic status epilepticus. This persisted despite levetiracetam, valproate, midazolam, phenobarbital, lacosamide, pregabalin, and propofol. Eventually, the patient was started on ketamine. Clinical seizures resolved but patient had persistent subclinical status epilepticus despite being at target temperature for 24 hours. MRI brain showed diffuse bilateral diffusion restriction, which confirmed acute anoxic brain injury. In light of the imaging findings and RSE, family elected for comfort care measures only. Patient expired four days after admission.
Patient 4
A 71-year-old woman with a history of hypertension, diabetes, congestive heart failure, chronic obstructive pulmonary disease, and previous cardiac arrest secondary to respiratory distress sustained recurrent cardiac arrest due to acute respiratory failure related to underlying lung disease and was admitted to the NSICU for TH. After target temperature was achieved, she had myoclonic status epilepticus with electrographic correlation. TH was maintained for 24 hours. She was treated with multiple antiepileptic drugs including levetiracetam, fosphenytoin, topiramate, lacosamide, midazolam, phenobarbital, ketamine, and pentobarbital. Despite resolution of clinical seizures, she continued to have subclinical seizures on cEEG. MRI brain was consistent with anoxic brain injury and family elected for comfort care measures. Patient expired 18 days after admission.
Patient 5
A 21-year-old man with a history of intermittent tachycardia experienced cardiac arrest and was subsequently admitted to the NSICU for TH. Initial workup was negative for pulmonary embolism or intoxication and neuroimaging showed early signs of cerebral edema. Persistent beating nystagmus was noted and cEEG revealed status epilepticus. He was treated with levetiracetam, midazolam, phenobarbital, and pentobarbital. Burst suppression was achieved and maintained for 48 hours. TH was maintained at target temperature for 24 hours. After re-warming, infusions were weaned without electrographic seizure recurrence. He remained comatose with non-reactive pupillary exam and MRI brain revealed laminar necrosis in bilateral cerebral hemispheres consistent with severe anoxic brain injury. His clinical course was complicated by pneumonia, acute renal failure requiring hemodialysis, and hypotension requiring infusion of multiple vasopressors. Ultimately, family elected for comfort care measures, and patient expired 17 days after admission.
Results
Two post-traumatic epilepsy patients with RSE became seizure free after completion of TH and remained seizure free after rewarming (Patient 1 and Patient 2). Both had good neurological recovery and were discharged for rehabilitation with eventual return to pre-admission functional status. Seizures persisted despite TH in two of three post-cardiac arrest patients (Patient 3 and 4), and all three expired. The results are summarized in Table 1.
![]()
Table 1: Summary of clinical data from the five patients
View Table 1
Discussion
Status epilepticus is a commonly encountered neurological emergency and is often refractory to initial treatments. A prospective study found 23% of patients in status epilepticus are refractory to first- and second-line therapy, and retrospective studies found this number was even higher (up to 43%) [9]. RSE is associated with a high morbidity and mortality (up to 30%) and additional treatment options should be sought [10]. TH is a promising adjunct therapy given the known antiepileptic and neuroprotective properties in both in vivo and in vitro studies [1,2,5-7]. However, it has not been systematically studied as treatment for RSE to know if the experimental results will translate into improved clinical outcomes for patients. In current clinical practice, TH is used as a last resort on a case-by-case basis when all other traditional treatments have been exhausted [8].
Data in the literature is extremely limited and heterogeneous. To our knowledge, there are currently nine published cases describing the use of TH in RSE, of which only five are adult patients [11-14]. RSE encompasses patients with a variety of medical comorbidities and seizure etiologies, which are likely directly linked to individual patient outcome and prognosis. The published adult cases represent this heterogeneity: two cases were related to limbic encephalitis, one case with hepatic encephalopathy, one case with acute ischemic stroke, and one additional cryptogenic case [12,13].
Here we have reported an additional five adult cases of RSE treated with TH: three with post-anoxic brain injury secondary to cardiac arrest and two related to remote traumatic brain injury. It is already known that status epilepticus post-cardiac arrest is a poor prognostic indicator, which is consistent with the three cases we report [3]. However, this starkly contrasts the results in the two post-traumatic epilepsy patients who had complete cessation of seizure activity without recurrence and minimal morbidity with TH. Both eventually returned to their pre-morbid functional capacities, which suggest the benefit of TH may be dependent on the etiology of the seizures and may have a more favorable outcome in post-traumatic epilepsy patients. There are obvious limitations in drawing conclusions from small numbers of cases. In addition, when studying status epilepticus it is difficult to both standardize treatments and separate treatments to isolate causative effects, making it impossible to determine if TH was the effective intervention and not the multiple medications initiated.
Conclusion
Patients with RSE caused by etiologies other than anoxic brain injury post-cardiac arrest may have better outcomes with TH, as supported by our two patients with RSE from post-traumatic epilepsy. These, to our knowledge, are the only two reported cases where TH was used in RSE from post-traumatic epilepsy and both had very favorable clinical outcomes, in contrast to the post-anoxic brain injury patients in this series. This suggests that the benefits of TH in RSE may be linked to the etiology of the seizures.
Many questions remain unanswered regarding TH. We do not know the patient population or under what conditions it is most beneficial. The variability in the cause of seizures and the implication to the overall medical condition of the patient affects the generalizability of the use and outcomes with TH. Optimal time to initiation of therapy, rate of cooling, target temperature, duration, and many other variables likely effect the benefit to risk ratio of TH and further controlled studies are needed to clarify these relationships. The etiology of RSE is a potential outcome predictor, which should be addressed in future prospective study designs for use of TH.
Disclosure
Jessica R. Fesler, Ryan Hakimi, Emmaculate M. Fields, and Andrea S. Hakimi declare that they have no conflict of interest. The authors received no financial support for the research, author, and/or publication of this article.
Acknowledgement
This case series was presented as a poster at the Neurocritical Care Society and American Academy of Neurology Annual Meetings in October 2013 and April 2014, respectively.
Ethical Statement
Institutional review board approval was obtained for this retrospective chart review.
Contribution Statement
Jessica Fesler MD obtained IRB approval, performed the chart review literature search and drafted the article. Ryan Hakimi DO, MS is the guarantor, responsible for concept, design, and revision of the manuscript. Emmaculate M. Fields APRN, CNP assisted with drafting the article and revision of the manuscript. Andrea Hakimi DO was responsible for review of EEG data and generation of figures as well as revision of the final manuscript.
References
-
Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, et al. (2010) Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci 32: 1912-1920.
-
D'Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, et al. (2013) Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann Neurol 73: 199-209.
-
Legriel S, Hilly-Ginoux J, Resche-Rigon M, Merceron S, Pinoteau J, et al. (2013) Prognostic value of electrographic postanoxic status epilepticus in comatose cardiac-arrest survivors in the therapeutic hypothermia era. Resuscitation 84: 343-350.
-
Wu TC, Grotta JC (2013) Hypothermia for acute ischaemic stroke. Lancet Neurol 12: 275-284.
-
Kowski AB, Kanaan H, Schmitt FC, Holtkamp M (2012) Deep hypothermia terminates status epilepticus--an experimental study. Brain Res 1446: 119-126.
-
Phillips KF, Deshpande LS, DeLorenzo RJ (2013) Hypothermia reduces calcium entry via the N-methyl-D-aspartate and ryanodine receptors in cultured hippocampal neurons. Eur J Pharmacol 698: 186-192.
-
Yu L, Zhou Y, Wang Y (2012) Effect of mild hypothermia on glutamate receptor expression after status epilepticus. Epilepsy Res 101: 56-69.
-
Fernandez A, Claassen J (2012) Refractory status epilepticus. Curr Opin Crit Care 18: 127-131.
-
Novy J, Logroscino G, Rossetti AO (2010) Refractory status epilepticus: a prospective observational study. Epilepsia 51: 251-256.
-
Young GB, Jordan KG, Doig GS (1996) An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 47: 83-89.
-
Orlowski JP, Erenberg G, Lueders H, Cruse RP (1984) Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med 12: 367-372.
-
Corry JJ, Dhar R, Murphy T, Diringer MN (2008) Hypothermia for refractory status epilepticus. Neurocrit Care 9: 189-197.
-
Cereda C, Berger MM, Rossetti AO (2009) Bowel ischemia: a rare complication of thiopental treatment for status epilepticus. Neurocrit Care 10: 355-358.
-
Elting JW, Naalt Jv, Fock JM (2010) Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol 14: 452-455.





