International Journal of Neurology and Neurotherapy
In Dubio Pro Therapia: Unexpected Recovery after Palliative Endoscopic Third Ventriculostomy and Temozolomide Chemotherapy in a Patient with Progressive Glioblastoma multiforme
Anne-Katrin Hickmann1*, Daniel Kirschenbaum2, Lucas Widmer3, MarcinSumila4, Stephan Wetzel5 and Robert Reisch1
1Center for Endoscopic and Minimally-Invasive Neurosurgery, Hirslanden Clinic, Switzerland
2Institute of Neuropathology, University Hospital Zürich, Switzerland
3Onkozentrum Hirslanden, Hirslanden Clinic, Switzerland
4Institute for Radiotherapy, Hirslanden Clinic, Switzerland
5Institute for Neuroradiology, Hirslanden Clinic, Switzerland
*Corresponding author:
Anne-Katrin Hickmann, Center for Endoscopic and Minimally Invasive Neurosurgery, Hirslanden Clinic, Witellikerstrasse 40, 8032 Zürich, Switzerland, Tel: +41 44 387 2859, Fax: +41 44 387 2855, E-mail: anne-katrin.hickmann@hirslanden.ch
Int J Neurol Neurother, IJNN-3-047, (Volume 3, Issue 2), Case Report; ISSN: 2378-3001
Received: February 18, 2016 | Accepted: April 15, 2016 | Published: April 18, 2016
Citation: Hickmann AK, Kirschenbaum D, Widmer L, Sumila M, Wetzel S, et al. (2016) In Dubio Pro Therapia: Unexpected Recovery after Palliative Endoscopic Third Ventriculostomy and Temozolomide Chemotherapy in a Patient with Progressive Glioblastoma multiforme. Int J Neurol Neurother 3:047. 10.23937/2378-3001/3/2/1047
Copyright: © 2016 Hickmann AK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A 38-year-old female with a 7-year history of recurrent high-grade astrocytoma was admitted to our institution presenting with progressive signs of increased intracranial pressure. MRI showed widespread tumor recurrence with diffuse infiltration of the ventricular walls, basal cisterns and concomitant hydrocephalus. After careful consideration a palliative endoscopic third ventriculostomy was performed. After surgery the patient's condition improved significantly. Thus far she had only undergone radiation, therefore now chemotherapy was initiated. After 4 courses of Temozolomide a follow-up MRI showed no pathologic contrast enhancement. Six months later the patient is still alive in good clinical condition (KPS 90%).
Keywords
Secondary Glioblastoma, Endoscopic ventriculostomy, Long-term survivor, Palliative
Introduction
Despite all therapies, the prognosis of high-grade astrocytomas is poor. Adjuvant therapies are known to prolong life, but so far no cure is available [1]. Occasionally the clinical course is less aggressive than expected and tumors respond surprisingly well to adjuvant treatment, which might be due to their histopathologic heterogeneity and their specific genetic profile [2].
The median age at diagnosis is 64 for glioblastomas and 45 years for anaplastic astrocytomas [1]. Especially the younger patients are often in the middle of their lives.
Just like our patient: the 32-year-old woman was 14 weeks pregnant when diagnosed with a malignant brain tumor, which greatly influences her approach towards treatment. Here we report her extraordinary clinical course.
Case Presentation
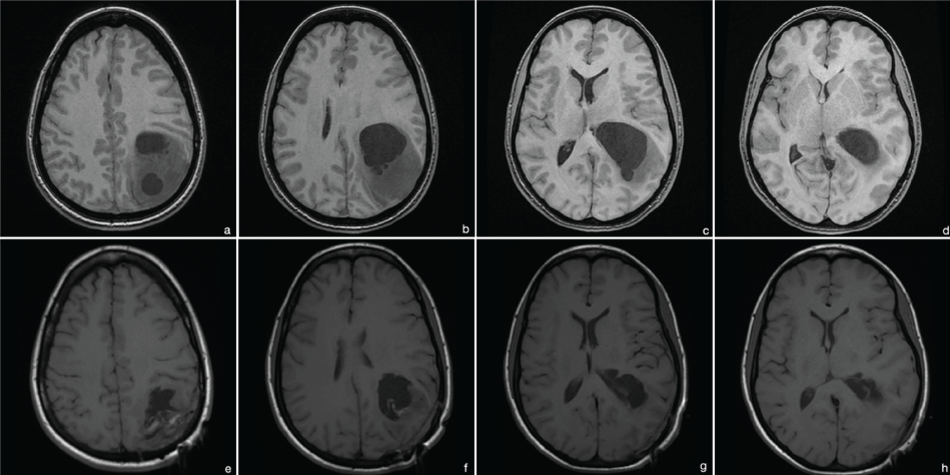
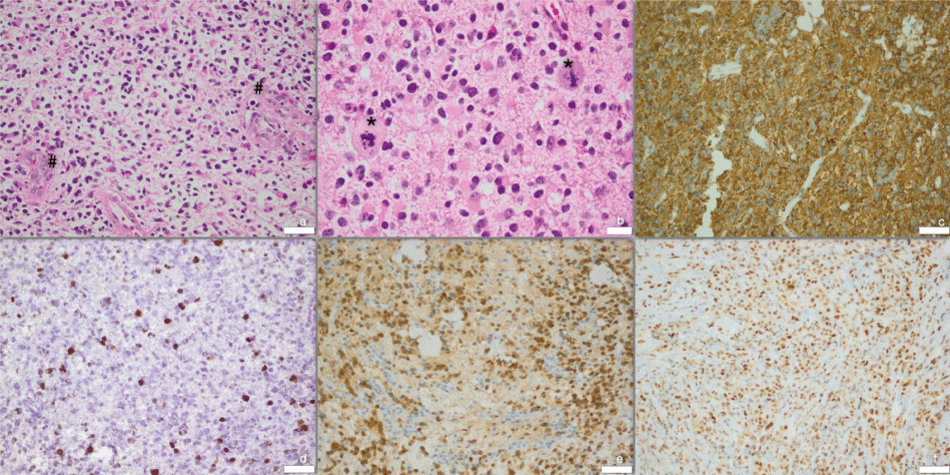
Due to intermittent dyslexia with dysphasia and progressive headache, an MRI was performed showing an inhomogeneous, partially cystic lesion in the left parieto-occipital lobe with surrounding edema and space occupying effect. No contrast agent was applied due to the patient's pregnancy (Figure 1). The first surgery for reduction of intracranial pressure and histopathological evaluation was performed in August 2009 while preserving the pregnancy. Histopathological evaluation showed a high-grade astrocytoma without clear-cut necrosis and with a Mib-1 proliferation rate of 15%. However glioblastoma multiforme was diagnosed, since definite vascular proliferations were seen (Figure 2(a, b & d) [3,4]. The standard combined radio-chemotherapy [5] was primarily postponed due to the patient's pregnancy, but lateron declined by the patient herself.
.
Figure 1: MRI 2009 (t1 without contrast due to pregnancy). (a-d) preoperative images: cystic, intraaxial lesion in the left pariteo-occipital lobe; postoperative images: partial tumor resection; (e-h) postoperative images showing partial tumor resection.
View Figure 1
.
Figure 2: Histology of surgical specimen from respective years. (a) 2009: HE-stain - vascular proliferation (#); (b) 2009: HE-stain - atypical mitoses (*); (c) 2014: GFAP; (d) 2009: MIB 15%; (e) 2014: IDH1; (f) 2014: ATRX; Scale bars for a, c, d, e, f 50 μm, for b 20 μm.
View Figure 2
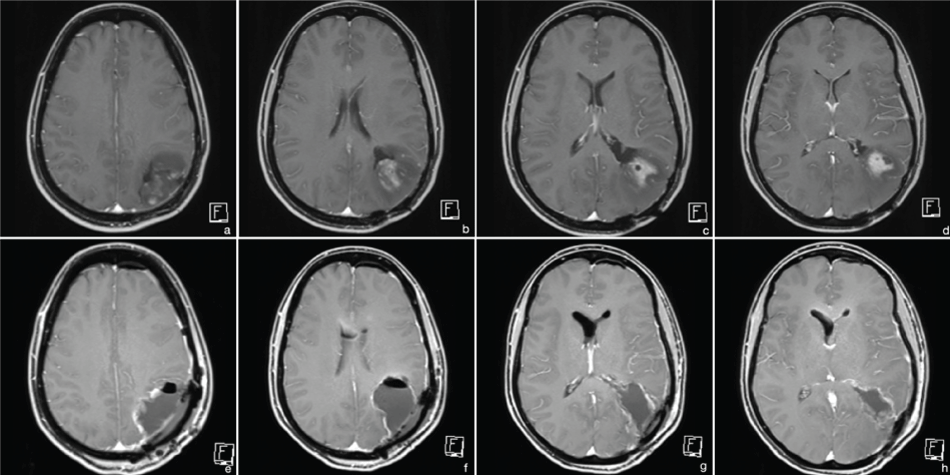
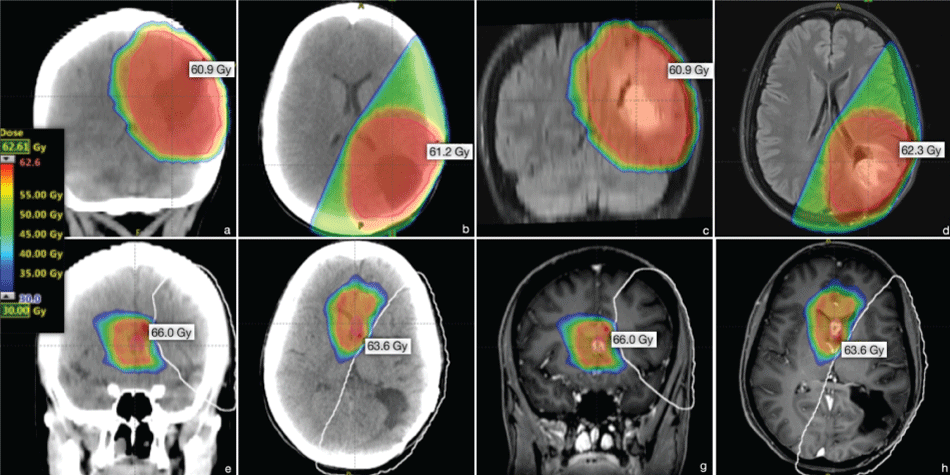
In 2011, growth of the residual tumor was noticed with inhomogeneous contrast-enhancement and re-operation was performed with complete resection (Figure 3). Histopathological evaluation showed recurrence of the high-grade astrocytic tumor. Standard radio-chemotherapy was recommended again. However, based on the patient's requests only radiation (RT) was performed. The former tumor bed region including a peripheral safety margin was defined as planning target volume. The patient was treated with conformal RT using three non-coplanar wedged 6 MV photon fields. The total dose was 60 Gy with 2Gy per fraction. Planar sections of the isodose plan are shown in figure 4(a-d).
.
Figure 3: MRI 2011 (t1 with contrast). (a-d) preoperative images - partially contrast enhancing lesion; (e-h) postoperative images - complete resection.
View Figure 3
.
Figure 4: Radiation Target Volume and isodose distribution (axial, coronal). (a, b) post-surgery CT projections (2011); (c, d) corresponding projections in the coregistered (fused) preoperative MRI (2011); (e, f) post-surgery CT projections (2015); (g, h) corresponding projections in the coregistered (fused) preoperative MRI (2015); (e-f) The bright white line marks the region which has received ≥ 20 Gy in the first course.
View Figure 4
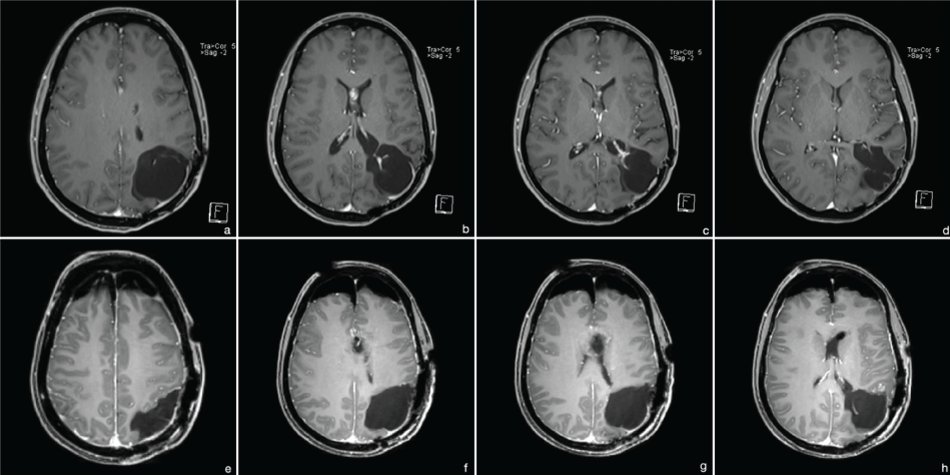
Follow-up MRI in 2014 showed stable conditions at the primary tumor site without signs of recurrence, but a new intraventricular manifestation. Complete resection was achieved using an endoscopic technique (Figure 5). Histopathological evaluation confirmed secondary glioblastoma WHO °IV, (IDH-1 positivity, retained nuclear ATRX, no loss of 1p19q, MGMT methylated, MIB-1 70%; Figure 2(c, e & f)). Adjuvant radio-chemotherapy with adapted radiation-volume was planned. The treatment planning volume including isodose distribution is displayed in figure 4(e-h). A volumetric modulated arc therapy (VMAT) with 6 MV photons for dose minimalization in the pre-irradiated brain region was applied. Due to side effects, the patient refused further treatment after only 3 radiation-sessions (6 Gy). Concomitant chemotherapy was rejected again.
.
Figure 5: MRI 2014 (t1 with contrast). (a-d) preoperative images - secondary intraventricular manifestation without signs of recurrence at the primary site; (e-h) postoperative images - complete resection.
View Figure 5
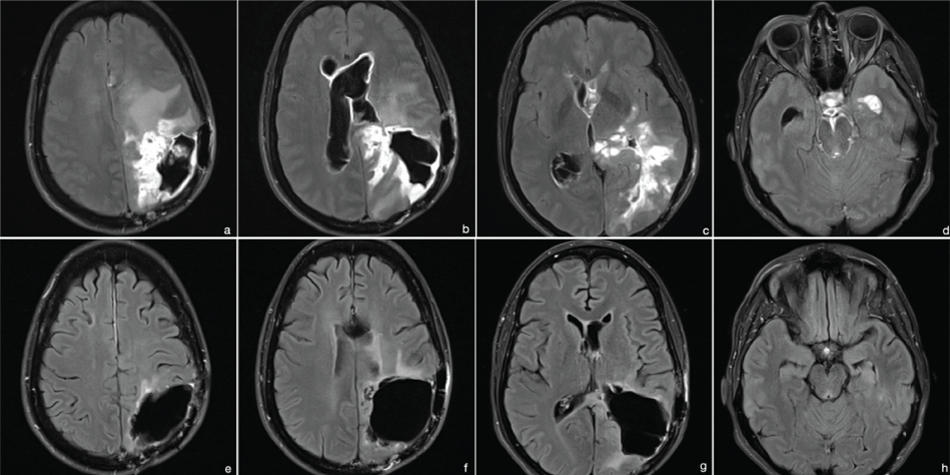
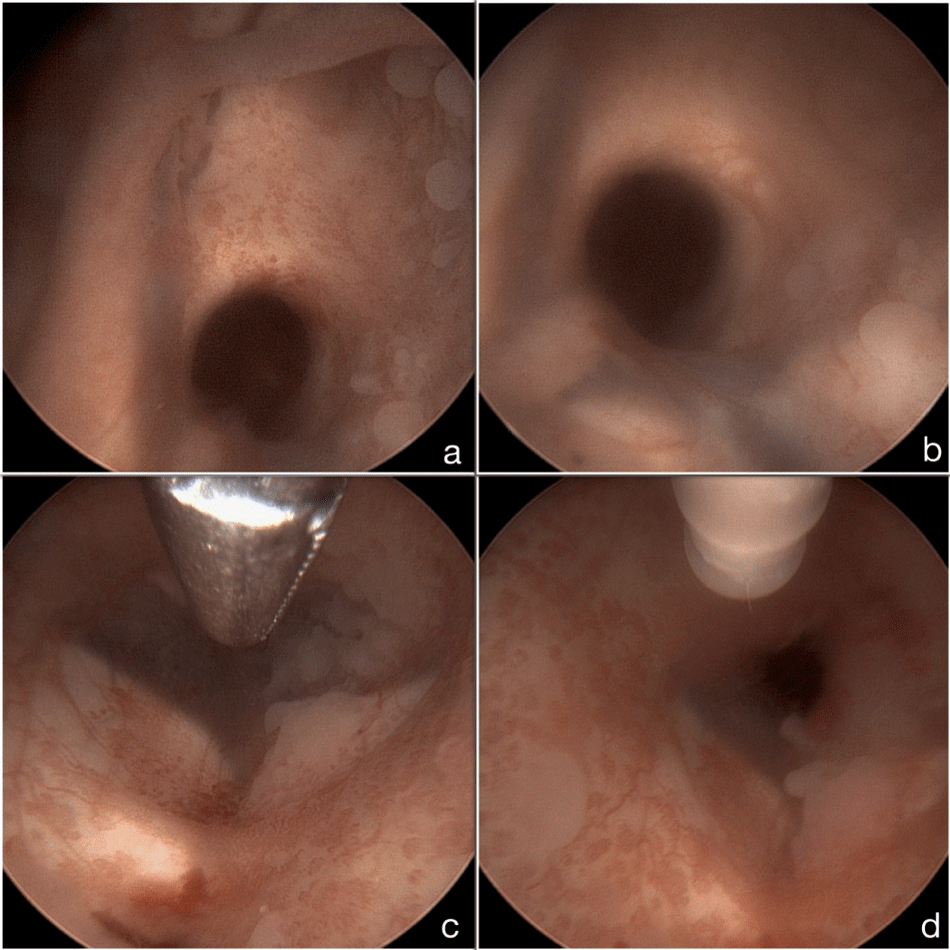
In May 2015, she was admitted with progressive headache and rapid deterioration of her vigilance. MRI revealed severe tumorprogression with diffuse infiltration of the ventricular walls and the basal cisterns (Figure 6(a-d)) with secondary, occlusive hydrocephalus. After thoroughly discussing treatment options and the patient's wishes with the family a palliative endoscopic third ventriculostomy (ETV) was performed (Figure 7, Supplemental Video). Pathological evaluation of the cerebrospinal fluid (CSF), collected during surgery, revealed multiple cells of the previously diagnosed glioblastoma, confirming diffuse tumor progression. Yet, the patient's clinical condition improved rapidly after surgery, leading to the assumption that her preoperative condition was mainly due to hydrocephalus and not tumor progression. Thus, despite the highly unfavorable prognosis chemotherapy with temozolomide was initiated (160-200 mg/m2 BSA, 6 cycles, 5/28). While undergoing therapy the patient's condition further improved. A follow-up MRI in September 2015, after 4 cycles of chemotherapy, showed marked regression of the tumor without any contrast enhancing tumor remnants (Figure 4 (e-h)). Six months after surgery the patient is still alive in good clinical condition (KPS 90%).
.
Figure 6: MRI 2015 (t2 flair with contrast). (a-d) preoperative images - diffuse infiltrative tumor growth with enlarged ventricles; (e-h) images after ETV and 4 cycles of temozolomide-treatment - tumor regression without pathologic contrast enhancement, normalized ventricular walls.
View Figure 6
.
Figure 7: Intraoperative Endoscopic Images. (a, b) Ventricular wall infiltration around Foramen of Monroi; (c) Floor of the 3rd ventricle before perforation; (d) Floor of the 3rd ventricle after perforation.
View Figure 7
Discussion
With increasing knowledge about genetic variations within astrocytomas, diverging clinical courses despite similar microscopic appearance can be explained [1,2,6-8]. This case, despite showing histopathological features of a glioblastoma °IV as defined by "The 2007 WHO Classification of tumors of the central nervous system" [3,4] diagnosed at two different pathological institutes, illustrates the difficulty with the existing classification. It has become known over the past years that molecular profiles might be more relevant towards prognosis than microscopic patterns [2,7] calling for a new classification.
Even though the tumor did presents with a favorable molecular profile (secondary malignisation (IDH1 pos), MGMT methylation, ATRX retained) [1,8] the effect of chemotherapy was not expected in this case. Yet, it shows that patients presenting with occlusive hydrocephalus and signs of raised intracranial pressure should be considered for treatment of the hydrocephalus according to the clinic's standard to allow for a differentiation of symptoms caused by hydrocephalus and those caused by tumorprogression itself, especially if further adjuvant treatment is still available. In cases of occlusive hydrocephalus, ETV poses an elegant option for treatment. The patient does not need prolonged external drainage with its associated risk of dislocation and infection and no foreign material needs to be placed as with a shunt. Furthermore, in cases of shunt placement, a high failure rate could be expected due to increased protein and cell count in the CSF.
One could argue that extensive therapy in a patient, who refused several treatments along the way, is not warranted. But due to the disease, her judgment may have been impaired as intracerebral tumors are known to cause cognitive deficits, which in turn influences treatment decisions [9,10], but may improve over time [11]. When being confronted with a life-threatening disease, maintaining quality of life is important and thus, some patients may refuse adjuvant treatment due to its side effects.
The response witnessed after chemotherapy might partially be due to the tumors favorable molecularprofile (MGMT methylated, IDH 1 positive) [1,2,8,12], but also due to its temozolomide naivety. Furthermore, the widespread infiltration may have lead to a vast breakdown ofthe blood-brain barrier, allowing for better penetration of the chemotherapeutic agent. This further underlines, if patients change their opinion towards treatment during the course of the disease and treatment options are still available, even controversial measures should be considered.
Conclusion
Patients with malignant brain tumors who present with tumor progression and coinciding hydrocephalus should be considered for treatment of the hydrocephalus in order to distinguish the causes of their deterioration, especially if standard treatment options are still available. Furthermore, ETV poses a smart technique for the treatment of occlusive hydrocephalus without the need for insertion of external drainage or a VP-shunt.
Acknowledgements/Funding
The radiation information was kindly revised by Dr. Jürgen Curshmann. No funding was received.
Video
Endoscopic Third Ventriculostomy: intraoperative impressions showing diffuse ventricular wall infiltration by tumor. 09-May-16
References
-
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359: 492-507.
-
Eder K, Kalman B (2014) Molecular heterogeneity of glioblastoma and its clinical relevance. Pathol Oncol Res 20: 777-787.
-
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97-109.
-
Feiden S, Feiden W (2008) [WHO classification of tumours of the CNS: revised edition of 2007 with critical comments on the typing und grading of common-type diffuse gliomas]. Pathologe 29: 411-421.
-
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
-
Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, et al. (2001) Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7: 839-845.
-
Aum DJ, Kim DH, Beaumont TL, Leuthardt EC, Dunn GP, et al. (2014) Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg Focus 37: E11.
-
Wick W, Weller M, Weiler M, Batchelor T, Yung AW, et al. (2011) Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro Oncol 13: 566-579.
-
Marson DC, Martin RC, Triebel KL, Nabors LB (2010) Capacity to consent to research participation in adults with malignant glioma. J Clin Oncol 28: 3844-3850.
-
Triebel KL, Martin RC, Nabors LB, Marson DC (2009) Medical decision-making capacity in patients with malignant glioma. Neurology 73: 2086-2092.
-
Flechl B, Ackerl M, Sax C, Dieckmann K, Crevenna R, et al. (2012) Neurocognitive and sociodemographic functioning of glioblastoma long-term survivors. J Neurooncol 109: 331-339.
-
Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, et al. (2000) Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343: 1350-1354.





