International Journal of Neurology and Neurotherapy
MR as a Biomarker for Metronidazole Induced Encephalopathy: Clinical, Neuroimaging and Differential Diagnostic Features
Charles D Donohoe* and Paul A Guidos
Department of Neurology, University of Missouri-Kansas City, USA
*Corresponding author:
Charles D Donohoe, Interim Chairman, Associate Professor of Neurology, Department of Neurology, Truman Medical Centers/UMKC School of Medicine, 2301 Holmes Street Suite 519, Kansas City, MO 64108, Tel: 816-404-5271, Fax: 816-404-4272, E-mail: Charles.Donohoe@tmcmed.org
Int J Neurol Neurother, IJNN-3-055, (Volume 3, Issue 4), Case Report; ISSN: 2378-3001
Received: May 27, 2016 | Accepted: July 30, 2016 | Published: August 27, 2016
Citation: Donohoe CD, Guidos PA (2016) MR as a Biomarker for Metronidazole Induced Encephalopathy: Clinical, Neuroimaging and Differential Diagnostic Features. Int J Neurol Neurother 3:055. 10.23937/2378-3001/3/4/1055
Copyright: © 2016 Donohoe CD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Central nervous system CNS toxicity is a rare complication of a commonly used antibiotic, metronidazole. We report a representative case of metronidazole-induced encephalopathy in an individual with increased vulnerability due to underlying liver disease [1]. Features included the essential clinical symptoms of dysarthria and ataxia and characteristic MR abnormalities of increased T2 and FLAIR signal in the dentate nuclei both of which were rapidly reversible upon drug discontinuation [2]. This relatively specific MR biomarker of metronidazole toxicity was indispensable in expediting the diagnosis in a critically ill patient in whom there were multiple differential diagnostic considerations including stroke.
Case Presentation
A 44-year-old white male was admitted for abdominal pain and ascites due to cirrhosis and end stage liver disease as the result of longstanding alcohol abuse. On admission, he was found to have pancreatitis with abnormal liver function tests including impaired coagulation (elevated INR) consistent with decompensated hepatic failure. He underwent paracentesis and was treated with spironolactone and furosemide. His initial neurologic examination was normal.
On hospital day 6, he complained of increasing abdominal pain. He was found to have an ileus on abdominal x-ray and Clostridium difficile assay was positive. A gastroenterology consultant prescribed IV Metronidazole 500 mg Q 8 hours and Vancomycin 125 mg Q 6 hours. On hospital day 18, psychiatry consultation was required for the emergence of agitation, anxiety and insomnia. On the morning of hospital day 20, he suddenly became severely dysarthric and ataxic to a degree where he was unable to stand or even sit without assistance. His speech was barely intelligible and swallowing was impaired requiring a feeding tube. A non-contrast computed tomograph CT brain was normal. Differential diagnostic considerations included Wernicke's and hepatic encephalopathy as well as a brainstem infarct.
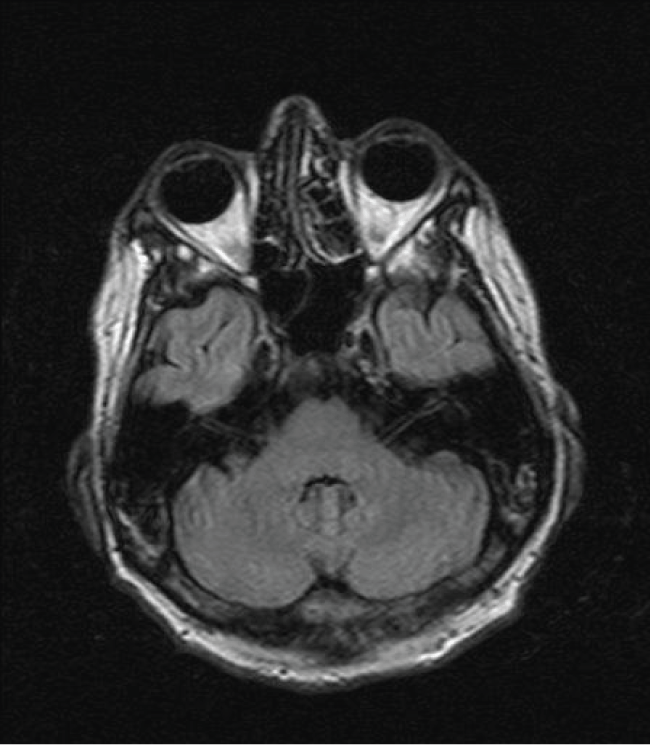
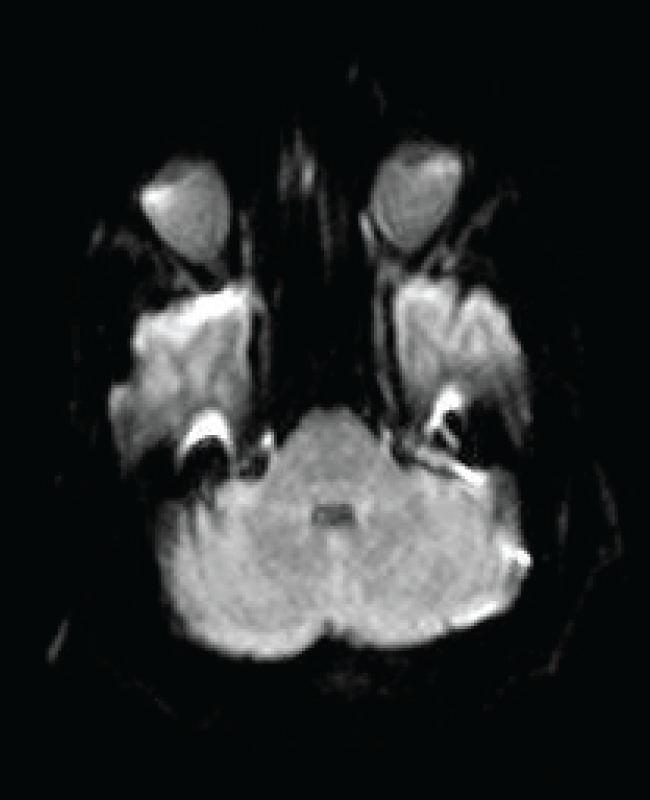
On hospital day 21, an MR brain demonstrated hyperintense signal on FLAIR (Figure 1) and T2 sequences in both cerebellar dentate nuclei, dorsal pons and central tegmental tract. Diffusion weighted images (DWIs) showed bright signal in both cerebellar dentate nuclei (Figure 2) with corresponding high apparent diffusion coefficient (ADC) consistent with vasogenic edema. The lesions did not demonstrate contrast enhancement.
.
Figure 1: Axial FLAIR image demonstrates bilateral symmetric hyperintense signal in the dentate nuclei (large arrow) of the cerebellum and both central tegmental tracts (small arrow).
View Figure 1
.
Figure 2: Axial diffusion weighted image (DWI) shows bright signal (restricted diffusion) in both dentate nuclei of the cerebellum (arrow). Apparent diffusion coefficient map (ADC) demonstrate a corresponding area of high ADC consistent with vasogenic edema.
View Figure 2
Neurology consultation found the patient to be alert, oriented with intact cognition but very dysarthric speech. Cranial nerve exam was significant for coarse horizontal nystagmus to both left and right lateral gaze. He was confined to bed and unable to feed himself because of incapacitating truncal and appendicular ataxia. Based on the characteristic MR findings of hyperintense signal most notable in both cerebellar dentate nuclei, a diagnosis of metronidazole-induced cerebellar toxicity was made. Metronidazole was promptly discontinued. Lumbar puncture was not performed due to an elevated INR secondary to his hepatic dysfunction.
Physical therapy and vestibular rehabilitation were initiated. By hospital day 34, nystagmus was no longer apparent but his speech, although improved, was still dysarthric. He was able to stand but required a roller walker to stabilize his gait. Repeat brain MR, 13 days after the initial MR, was normal (Figure 3 and Figure 4).
Discussion
Metronidazole is a widely used antibiotic agent for anaerobic and parasitic infection. It is the treatment of choice in moderate uncomplicated Clostridium difficile infections generally without significant toxicity. Common side effects include nausea, dry mouth, and diarrhea. Rare are significant neurotoxic side effects of metronidazole. The most commonly seen neurologic complication of metronidazole toxicity involves the peripheral nervous system predominantly as a sensory peripheral neuropathy [3].
Patients with metronidazole central nervous system (CNS) toxicity most commonly present with dysarthria and ataxia. However, symptoms may also include altered mental status, seizures, and visual disturbances [1]. MR is the modality of choice in identifying CNS toxicity. Structures most vulnerable to metronidazole toxicity include both cerebellar dentate nuclei but also the midbrain (tectum, red nucleus, tegmentum around the periaqueductal gray matter), dorsal pons, superior olivary nucleus and the splenium of the corpus callosum [4].
On FLAIR and T2 weighted images, common findings include hyperintense lesions of the cerebellar dentate nuclei, midbrain, dorsal pons, corpus callosum, and cerebral white matter. A pattern consistent with vasogenic edema is usually identified with bright signal on diffusion-weighted images (DWI) and high apparent diffusion coefficient (ADC). These MR findings are most conspicuous in bilateral cerebellar dentate nuclei and resolve in conjunction with clinical improvement upon drug discontinuation. Permanent residual neurologic deficits have been reported highlighting the need for prompt recognition.
The nature of the neurotoxic effects of metronidazole is unknown. Postulated mechanisms include axonal swelling with interstitial edema due to interference of microsomal metabolism resulting in energy deprivation [5]. Cases have been reported when serum levels were in the therapeutic range. Findings on MR spectroscopy suggest the possibility of reversible mitochondrial dysfunction (increased lactate peak) in susceptible individuals [6]. The practical point is that despite a lack of clarity as to a basic mechanism, both the MR and the clinical abnormalities are rapidly reversible [7].
Within 2 weeks of the onset of symptom and discontinuation of metronidazole, the MR changes completely resolved and his clinical symptoms improved considerably. As in our patient most metronidazole induced CNS toxicity presents with cerebellar symptoms of ataxia and dysarthria. One third exhibited altered mental status and 15% presented with seizures. Basically all patients with cerebellar dysfunction had abnormalities on MR.
Follow up MRs demonstrated complete resolution of these abnormalities in 83% of patients. The median duration of metronidazole exposure in a review of case series was 54 days although in 26% toxicity had emerged in less than a week and in 11% in less than 72 hours. Although generally seen with longer courses of treatment, toxicity can present in the wide range of cumulative doses from 25-1080 grams [8].
Metronidazole crosses the blood-brain barrier and achieves therapeutic concentrations in the cerebrospinal fluid. MR defines bilateral involvement with axonal swelling and increased water content suggestive of a toxic-metabolic process [7]. Although there has been no direct correlation between blood levels and clinical toxicity, in patients with advanced liver disease the clearance of metronidazole is reduced to 35% and the half-life extended to 280% of healthy control subjects. Beyond liver disease there is an increased incidence of metronidazole toxicity in chronic renal failure, diabetes, hematologic and solid organ malignancies [9].
The dentate is the largest of the cerebellar nuclei and plays an integral role in the coordination of voluntary movements. It is highly cellular and metabolically active as is the cerebellum in general which accounts for only 10% of brain volume but contains over 50% of the total neurons in the brain. The susceptibility of the dentate and dorsal brainstem nuclei is not understood but similar MR changes have been identified in maple syrup urine disease, enteroviral encephalopathies, nonalcoholic Wernicke's encephalopathy and with other antibiotics such as isoniazid and cycloserine [10].
Isolated cases have been reported in conjunction with occupational exposure to industrial toxins such as methyl bromide and methyl iodide [11]. These diverse toxic, genetic and nutritional disorders been linked as 'energy deprivation syndromes' with a shared toxicity that appears to be related to disruption of metabolic pathways involved in basic glycolysis and pyruvate oxidation [12].
Conclusion
Metronidazole, a commonly used antibiotic in routine and critical care settings, infrequently causes central nervous system toxicity presenting as a cerebellar syndrome including dysarthria and ataxia with or without accompanying encephalopathy. Symptoms can evolve acutely as a posterior circulation stroke mimic. Underlying diabetes, hepatic and renal dysfunction and poor nutritional status associated with chronic illness appear as major predisposing factors. Rapid correlation of the clinical and neuroimaging is critical in expediting the diagnosis. Prompt cessation of metronidazole is usually associated with an excellent clinical prognosis and rapid resolution of the MR abnormalities. In our case clinical improvement laged behind resolution of the MR abnormalities.
We report our case to highlight this valuable MR biomarker linking an antibiotic (metronidazole) to a characteristic clinical syndrome (dysarthria and ataxia) to a relatively specific MR abnormality (increased signal on T2 and FLAIR in both dentate nuclei of the cerebellum). However, bilateral hyperintense MR signal on T2 and FLAIR in the dentate nuclei is not entirely unique to metronidazole and may serve as a more general biomarker of neurotoxicity due other drugs, nutritional deficiency states or occupational chemical exposure.
References
-
Ahmed A, Loes DJ, Bressler EL (1995) Reversible magnetic resonance imaging findings in metronidazole-induced encephalopathy. Neurology 45: 588-589.
-
Bottenberg MM, Hegge KA, Eastman DK, Kumar R (2011) Metronidazole-induced encephalopathy: a case report and review of the literature. J Clin Pharmacol 51: 112-116.
-
John P Knorr, Imran Javed, Neha Sahni, Ceylan Z Cankurtaran, and Jorge A Ortiz (2012) Metronidazole-Induced Encephalopathy in a Patient with End-Stage Liver Disease. Case Reports Hepatol 2012: Article ID 209258.
-
Kim E, Na DG, Kim EY, Kim JH, Son KR, et al. (2007) MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol 28: 1652-1658.
-
Loft S, Sonne J, Døssing M, Andreasen PB (1987) Metronidazole pharmacokinetics in patients with hepatic encephalopathy. Scand J Gastroenterol 22: 117-123.
-
Cecil KM, Halsted MJ, Schapiro M, Dinopoulos A, Jones BV (2002) Reversible MR imaging and MR spectroscopyabnormalities in association with metronidazole therapy. J Comput Assist Tomogr 26: 948-951.
-
Peter P, John M (2014) Isoniazid-induced cerebellitis: a disguised presentation. Singapore Med J 55: e17-19.
-
Kato H, Sosa H, Mori M, Kaneko T (2015) Clinical Characteristics of Metronidazole-induced Encephalopathy: A report of Two Cases and a Review of 32 Japanese Cases in the Literature. Kansenshogaku Zasshi 89: 559-566.
-
Patel K, Green-Hopkins I, Lu S, Tunkel AR (2008) Cerebellar Ataxia following Prolonged Use of Metronidazole: Case Report and Literature Review. Int J Infect Dis 12: 111-114.
-
Kim S, Kang M, Cho JH, Choi S (2014) Reversible magnetic resonance imaging findings in cycloserine- induced encephalopathy: A case report. Neurology Asia 19: 417-419.
-
Geyer HL, Schaumburg HH, Herskovitz S (2005) Methyl bromide intoxication causes reversible symmetric brainstem and cerebellar MRI lesions. Neurology 64: 1279-1281.
-
Lekhra GP, Sonali J, Maheshwari A, Rathore Y (2013) Reversible Hyperintense Dentate Lesions. Journal of Evolution of Medical and Dental Sciences 2: 2763-2764.