Hypertension disorders complicate up to 10%-11% of all pregnancies and remain leading causes of poor outcome, including placental abruption, organ failure, cerebrovascular accident and disseminated intravascular coagulation. These disorders are also associated with increased risk of perinatal death, fetal intrauterine growth restriction, and prematurity/preterm delivery.
Epidemiological evidences supporting the worse prognosis associated with hypertension in pregnancy provide a strong basis for developing perinatal morbidity and mortality risk prediction models.
Of the many risk markers for hypertensive disorders, some are known at booking and increase the risk of hypertensive disorders two- to four-fold. They include pre-existing hypertension, diabetes mellitus and renal disease, previous preeclampsia, antiphospholipid antibody syndrome, overweight/obesity, inter-pregnancy interval ≥ 10 years, and multiple pregnancies.
Recently, the additive value of some instrumental techniques (including uterine artery Doppler velocimetry, electrocardiography and ambulatory blood pressure monitoring) and their combinations with maternal factors and biochemical markers to refine risk stratification for hypertensive disorders in pregnancy has also been evaluated.
The main aim of our systematic review was to summarize the present state of knowledge in this active area of broad interest. Specifically, we aimed to provide an overview of recent contributions on the role of electrocardiography for the identification of women at increased risk of hypertensive complications during pregnancy.
Briefly, current Guidelines recommend performing a 12-lead electrocardiogram in order to evaluate the presence of left ventricular hypertrophy in pregnant women. Nevertheless, some abnormal electrocardiographic patterns, particularly in the first trimester of pregnancy, may increase the risk of maternal and neonatal complications. In this context, left atrial abnormalities in lead V1 have been suggested as independent predictors of hypertensive disorders and other pregnancy complications including fetal growth restriction, HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, placental abruption, stillbirth, premature delivery and neonatal death.
Available data support the notion that risk stratification for hypertensive disorders might be improved in the first-trimester of pregnancy using standard electrocardiography in combination with maternal characteristics and history. An effective screening for hypertensive disorders is useful to identify women that would potentially benefit from a closer surveillance and from prophylactic pharmacological interventions.
Electrocardiography, Pregnancy, Hypertension, Eclampsia, Pre-eclampsia, Hypertensive disorders, Prognosis
ABPM: Ambulatory Blood Pressure Monitoring; BP: Blood Pressure; CI: Confidence Interval; ECG: Electrocardiography; HELLP: Hemolysis, Elevated Liver Enzymes, Low Platelets; HR: Heart Rate; MAP: Mean Arterial Pressure; OR: Odds Ratio; RAS: Rennin Angiotensin System; SOGC: Society of Obstetricians and Gynecologists of Canada
As recommended by the National High Blood Pressure (BP) Education Program Working Group on High BP in Pregnancy [1], hypertensive disorders during pregnancy are classified into 4 categories:
• Chronic hypertension
• Preeclampsia-eclampsia
• Preeclampsia superimposed on chronic hypertension
• Gestational hypertension (transient hypertension of pregnancy or chronic hypertension identified in the latter half of pregnancy).
In 2014, the Society of Obstetricians and Gynecologists of Canada (SOGC) [2] released revised guidelines that simplified the classification of hypertension in pregnancy into the following categories: (i) Preexisting hypertension; (ii) Gestational hypertension; (iii) Preeclampsia; and (iv) "Other hypertensive effects" on the basis of different diagnostic considerations.
Determining the true incidence of the hypertensive disorders of pregnancy is complicated by variations in the reported classification of the disorders. The most commonly cited and accepted estimate of hypertensive disorder of pregnancy occurrence is around 10-11% [3].
Hypertensive disorders of pregnancy can develop during pregnancy or delivery and are major causes of maternal and perinatal morbidity and mortality [4-6]. Specifically, hypertensive disorders can trigger some severe forms of maternal complications, such as cardiovascular and cerebrovascular diseases, liver and kidney failure, placental abruption, disseminated intravascular coagulation and HELLP (Hemolysis, Elevated Liver Enzymes, Low Platelets) syndrome. Under these circumstances, the placenta dysfunction may occur, leading to fetal growth restriction, fetal distress, preterm birth, intrauterine fetal demise, stillbirth and neonatal asphyxia [1]. Moreover, hypertensive disorders of pregnancy are associated with increased risk of future chronic hypertension [4-7].
Over the past few decades, several studies have been conducted to identify the pregnant women at higher risk of hypertensive disorders.
This review summarizes the present state of knowledge in this active area of broad interest. Specifically, we aimed to provide an overview of recent contributions on the role of instrumental techniques for the identification of women at increased risk of hypertensive complications during pregnancy.
To this purpose, we searched for clinical studies and systematic overviews using research methodology filters [8,9]. The following research terms were used: "hypertension", "pregnancy", "gestational hypertension", "eclampsia", "pre-eclampsia", "blood pressure" and prognosis". We also checked the reference list of identified articles and previous systematic reviews to find other relevant studies.
Traditionally, diagnosis and management of arterial hypertension are based on BP measurements taken in the physician's office. Women should be allowed to sit quietly for 5-10 minutes before each BP measurement [1,2]. Of note, compression on the inferior vena cava by the gravid uterus while the patient is supine can alter readings substantially, leading to an underestimation of the BP.
Similarly, BP measured in the left lateral position may yield falsely low values if the BP is measured in the higher arm, unless the cuff is carefully maintained at the level of the heart. Thus, BP should be measured in the sitting position, with the cuff at the level of the heart. Korotkoff sounds I (the first sound) and V (the disappearance of sound) should be used to denote the systolic BP and diastolic BP, respectively. Maternal systolic BP greater than 160 mmHg or diastolic BP greater than 110 mmHg denotes severe disease [1,2].
Home BP measurements are recommended in the pregnant population. In this context, some automated BP devices have been validated in pregnancy (http://www.dableducational.org/sphygmomanometers/devices_1_clinical.html#ClinTable). We strongly recommend using validated devices for assessment of hypertension severity and control during pregnancy.
A large clinical study involving 9149 women with singleton pregnancies examined the performance of screening for hypertensive disorders in pregnancy comparing systolic BP, diastolic BP, and Mean Arterial Pressure (MAP) measured by validated automated devices. Specifically, the best performance in screening was provided by MAP [10]. The detection rate of early-preeclampsia at a 10% false-positive rate increased from 47% in screening by maternal factor-derived a priori risk alone to 76% in screening by its combination with MAP. The respective detection rates for late-pre-eclampsia increased from 41 to 52% and for gestational hypertension increased from 31 to 48% [10].
Epidemiological evidence supporting the worse prognosis associated with hypertension in pregnancy provides a strong basis for developing risk prediction models to identify women at increased risk for hypertensive disorders. These women may require a closer surveillance and preventive treatments [11].
To date, several risk factors for development of hypertensive disorders in pregnancy have been described, including preexisting chronic hypertension, chronic vascular and renal disease, connective tissue disease, dyslipidemia, diabetes, obesity, age more than 40 years, multifetal gestation, family history of preeclampsia and fetal growth restriction [7,12-20].
To improve the sensitivity and the positive predictive values of screening programs, some biochemical markers (including laboratory markers, urinary proteomics, markers of inflammation, antiphospholipid antibodies, and coagulation factors) have been investigated as potential predictors of hypertensive disorders during pregnancy [7,21-24].
Particularly, an experimental study including 120 normotensive pregnant and 60 pregnancy-induced hypertensive women reported that pregnancy-induced hypertension was associated with significantly lower levels of serum total calcium, urinary calcium and magnesium excretions and plasma renin activity [25].
To further clarify these findings, a systematic overview and meta-analysis investigated the relationship between serum zinc, magnesium, and calcium levels and pregnancy-induced hypertension [26].
Briefly, a total of 14 studies were included and results indicated that patients with pregnancy-induced hypertension had lower serum zinc, calcium, and magnesium concentration than healthy gravidas [26].
Such results have some implications on the aetiology of hypertension in pregnancy and suggest that mineral supplementation during the antenatal period may influence prevention and treatment of hypertensive disorders of pregnancy.
Although several circulating and urinary markers have been proposed as potential predictors of hypertensive disorders during pregnancy, any single test may lack predictive value or practical utility to be applied at large [7].
Moreover, early prediction of hypertensive disorders in healthy and initially normotensive pregnant women remains problematic, partly because severe forms such as pre-eclampsia and eclampsia are etiologically complex and heterogeneous conditions [7,11].
In this context, evidence suggests that an effective screening for the development of hypertensive disorders can be provided in the first-trimester of pregnancy. Instrumental/imaging techniques might improve the accuracy of multivariable predictive models for the prediction of hypertensive disorders of pregnancy, especially for the more severe forms. Particularly, screening by a combination of maternal risk factors, uterine artery Doppler, out-of-office BP measurements and standard Electrocardiography (ECG) can identify women at increasing risk for the development of hypertensive complications (Figure 1) [7].
 Figure 1: Instrumental techniques used to identify women at increased risk of hypertensive disorders during pregnancy. For each test, main parameters tested as predictors of hypertensive disorders are reported [7]. View Figure 1
Figure 1: Instrumental techniques used to identify women at increased risk of hypertensive disorders during pregnancy. For each test, main parameters tested as predictors of hypertensive disorders are reported [7]. View Figure 1
Ambulatory BP Monitoring (ABPM) has become a clinically useful modality in BP assessment in pregnancy; efforts have been made to predict hypertensive disorders of pregnancy by using ABPM [7].
In a prospective cohort study by Bellomo, et al. 254 women without preexisting hypertension and not treated with antihypertensive drugs aid with high (n = 148) or normal (n = 106) office BP underwent 24-hour noninvasive ABPM [27]. The Authors showed that in women with elevated BP during their third trimester of pregnancy, 24-hour BP was superior to office BP to predict the outcome. The sensitivity and specificity of 24-hour BP were 87.5% and 77.7%; for office BP measurement, 91.6% and 55.4%; for 24-hour proteinuria, 47.2% and 100%, respectively, for the prediction of pre-eclampsia [27].
More recently, the usefulness of ABPM during pregnancy was confirmed by Brown, et al. Specifically, 122 pregnant women who were considered at high risk for the development of pre-eclampsia underwent 24-hour ABPM between 18 and 30 weeks gestation, while their condition was normotensive according to routine mercury sphygmomanometry [28]. One hundred sixty-four healthy primigravid women who were considered at usual risk for preeclampsia underwent the same tests as a parallel study [28]. Routine BP, awake and sleep average BP, and 24-hour mean average BP were entered into multiple logistic regression as predictors of either preeclampsia or gestational hypertension; significant variables were then tested by a series of receiver operator curves. Results showed that awake and sleeping BP was higher in mid-pregnancy in women who later developed preeclampsia or gestational hypertension [28]. In particular, hypertension during sleep was a common finding in women with hypertensive disorders of pregnancy. These women also showed higher awake BP and a greater frequency of adverse maternal and fetal outcomes [29].
Furthermore, Hermida, et al. [30] have shown that, in pregnancy, the hyperbaric index (area of BP excess above the upper limit of a time-specified tolerance interval) derived from ABPM was superior to office measurements for predicting the outcome of pregnancy.
Normal placentation is achieved through successful trophoblast invasion of the maternal decidua and myometrium via the dilated spiral arteries. In the process, a low resistance vascular bed with a high blood flow is created. Physiological changes during pregnancy convert the spiral arteries from small muscular arteries to dilated uteroplacental vessels, which are able to accommodate the hemodynamic forces of pregnancy. Unsuccessful trophoblast invasion, with consequent under perfusion of the placenta, leads to the release of hormones into the maternal circulation which is believed to be the underlying mechanism for the development of hypertensive disorders [7,31-33].
The use of Doppler imaging permits non-invasive evaluation of the uteroplacental circulation by comparing systolic and diastolic waveforms. In this context, the assessment of uteroplacental circulation by Doppler ultrasonography of the uterine arteries has been reported in numerous studies as a promising technique for predicting the level of risk for hypertensive disorders in pregnancy [34-37].
A systematic review and meta-analysis [37] of studies in which Doppler assessment of the uterine arteries was used, showed that abnormal uterine artery waveform was a strong predictor of preeclampsia. In particular, an increased pulsatility index with notching was an independent predictor of pre-eclampsia with a positive likelihood ratio equal to 21.0 among high-risk patients and 7.5 among low-risk patients. It was also a predictor of overall (positive likelihood ratio 9.1) and severe (positive likelihood ratio 14.6) intrauterine growth restriction among low-risk patients [37].
Pregnancy may induce some ECG changes which may regress later in pregnancy or following delivery [7,38-48]. Main ECG changes induced by pregnancy are reported in Figure 2.
 Figure 2: ECG changes in normal pregnancy [48]. Heart rate increases progressively throughout the pregnancy, reaching a peak during the third trimester. Gestational age also impacts QRS complex and T waves, promoting a leftward axis shift as pregnancy progresses. PR interval exhibits a significant reduction in the mean values during pregnancy, while the QRS amplitude generally increases slightly in the late pregnancy. View Figure 2
Figure 2: ECG changes in normal pregnancy [48]. Heart rate increases progressively throughout the pregnancy, reaching a peak during the third trimester. Gestational age also impacts QRS complex and T waves, promoting a leftward axis shift as pregnancy progresses. PR interval exhibits a significant reduction in the mean values during pregnancy, while the QRS amplitude generally increases slightly in the late pregnancy. View Figure 2
Some studies have investigated the ECG changes in pregnant women with hypertensive disorders during pregnancy [39,47-51]. To date, there is evidence that hypertensive disorders of pregnancy can be predicted by changes in P-wave morphology and QT interval [39,48].
QT interval seems to be unaffected by normal pregnancy [38]. Conversely, pregnancies with abnormal uterine perfusion that developed pathological outcomes seem to be paralleled by changes in ventricular repolarization that may precede clinical symptoms [50].
In this context, a prospective study by Isezuo and Ekele [49] including 60 pregnant Nigerian women (mean age 19.5 ± 4.2 years) showed that eclamptic patients had higher frequency of abnormal QTc (46.7% versus 6.6%, odds ratio [OR]: 9.2; 95% Confidence Interval [CI]: 1.61-68.48, p = 0.01) as measured on the surface ECG when compared to women with normal pregnancy [49].
Similarly, Raffaelli and co-workers [51] evaluated the effect of pre-eclampsia on electrical cardiac activity on Caucasian women from Italy. They demonstrated that pregnancies complicated by pre-eclampsia had a significant alteration of ventricular repolarization. They compared pre-partum ECGs of 76 consecutive pre-eclamptic women with those of 76 healthy pregnant women. All of the routine ECG parameters were considered, and ventricular repolarization was assessed by QT interval and QT dispersion (QTd).
Pre-eclamptic women showed a lower heart rate (HR, 77.4 ± 14.3 vs. 81.6 ± 11.0 beats per minute [bpm]), a longer mean QTc interval (442.7 ± 26.7 vs. 423.7 ± 20.7 msec) and a higher QTd (24.0 vs. 22.0 msec) than the control group [51]. Moreover, P-wave duration was significantly longer in the pre-eclamptic women than in the control group of normal pregnancy [51].
Just recently, a case-control study from Kirbas and co-workers [52] evaluated P wave parameters to determine the association between preeclampsia and future cardiovascular risk and to study the possible correlation between P waves and severity of preeclampsia among women enrolled between January and July 2014 at Zekai Tahir Burak Women's Health Training and Research Hospital of Turkey. Specifically, maximum (Pmax) and minimum (Pmin) P-wave durations were defined as the longest and shortest measurable P-wave durations, respectively, in any lead. P-wave dispersion (Pd) was calculated as the maximum minus minimum P-wave duration [52]. They demonstrated that the Pd values of the severe preeclampsia group (mean age 28.5 ± 5.6) were significantly higher compared to that of the mild preeclampsia group (mean age 28.2 ± 5.5) [52].
These findings are consistent with a recent prospective collaborative screening study between gynecologists, internists and cardiologists [47,48] from Italy, investigating the potential additive role of standard ECG in the identification of Caucasian women at increased risk for hypertensive complications [47].
P wave morphology was analyzed in all of the standard leads. The criteria used for the diagnosis of P wave abnormality in lead V1 were: (1) Bipeak interval in deeply notched P wave with (2) Terminal forces equal to or more negative than -0.04 mm•sec, as obtained from the product of the depth of the terminal negative deflection and its duration [53,54]. The following other criteria were used for the diagnosis of left atrial abnormality in any other lead than V1: (1) Bipeak interval in deeply notched P waves wider than 0.04 sec or (2) P-wave/PR-segment ratio greater than 1.6 or (3) P wave higher than 3 mm or (4) Total P wave duration greater than 0.11 sec [53,54]. The primary outcome of the study was the development of gestational hypertension, pre-eclampsia and eclampsia. The secondary outcome was a composite measure of hypertensive disorders and other pregnancy complications including fetal growth restriction, HELLP syndrome, placental abruption, stillbirth, premature delivery and neonatal death [1,55,56]. A total of 221 pregnant women were included in the final analysis [47]. Gestational hypertension occurred in 22 women, 5 women experienced pre-eclampsia (3 of these developed HELLP syndrome) and 1 woman had eclampsia. The secondary composite outcome was recorded in 43 women. Multiple events (hypertensive disorders and other maternal or fetal/neonatal complications) were observed in 9 women.
Overall, premature deliveries occurred in 14 women, 6 women delivered growth-restricted neonates, 2 women experienced placental abruption and 2 congenital heart defect requiring admission to neonatal nursery were recorded. At entry, left atrial abnormality in lead V1 was more prevalent in women with hypertension disorders (p = 0.002). Age, laboratory tests, HR and other ECG parameters including QT interval and left atrial abnormality observed in other leads than V1 did not differ between the two groups. In a multivariable model, MAP and left atrial abnormality in lead V1 were independent predictors of hypertensive disorders. In particular, the presence of left atrial abnormality in lead V1 was associated to a 4-fold increased risk of developing hypertensive disorders (OR: 4.35; 95% CI: 1.84-10.31; p = 0.001).
Of note, the same prediction model also proved significance to identify pregnant women at increased risk for the occurrence of maternal and fetal/neonatal complications [47].
Abnormality of P-wave morphology in lead V1 tested as predictor of hypertensive disorders during pregnancy [47] is commonly used as an ECG sign of left atrial enlargement and it may be easily diagnosed by traditional visual interpretation of ECG tracings, without any need of digitalization or other computer facilities [7].
The mechanisms linking left atrial abnormality on ECG with hypertensive disorders are still elusive [48]. However, several mechanisms, possibly reflected by abnormal left atrial activation on ECG has been suggested. These include increased reactivity to angiotensin II and up-regulation of angiotensin type 1 receptors, with activation of auto-antibodies targeting these receptors [48].
Finally, left ventricular hypertrophy has been suggested to mediate the relation between hypertension and left atrial enlargement [57].
The presence of abnormal P wave morphology at ECG may be a marker of abnormal left ventricular mass which commonly develop in both early and late onset preeclampsia [58-61].
In this context, a recent retrospective study of pregnant women with chronic hypertension [62] showed that women with left ventricular hypertrophy were at greater risk to be delivered preterm (p = 0.001), to develop superimposed preeclampsia (p = 0.028), and to have an infant requiring intensive care (p = 0.023) when compared with those without ventricular hypertrophy. Notably, these findings persisted after adjustment for age, race, and parity [62].
Nevertheless, echocardiographic data on the relationship between the simultaneous adaptations of left ventricle and left atrium during hypertensive disorders of pregnancy are scarce and conflicting [63-65].
At present, there is considerable interest for prevention of hypertensive disorders of pregnancy, for which our therapeutic approaches are still limited.
Some reports on routine antenatal care suggest that a woman's level of risk for hypertensive disorders may be quantified [7]. In this context, an effective screening for hypertensive disorders might be achieved in the first-trimester of pregnancy using standard ECG in combination with maternal characteristics and history [7,39,47,48].
To better understand potential clinical implications of ECG, we propose an algorithm for specialist referral based on observations from recent studies (Figure 3).
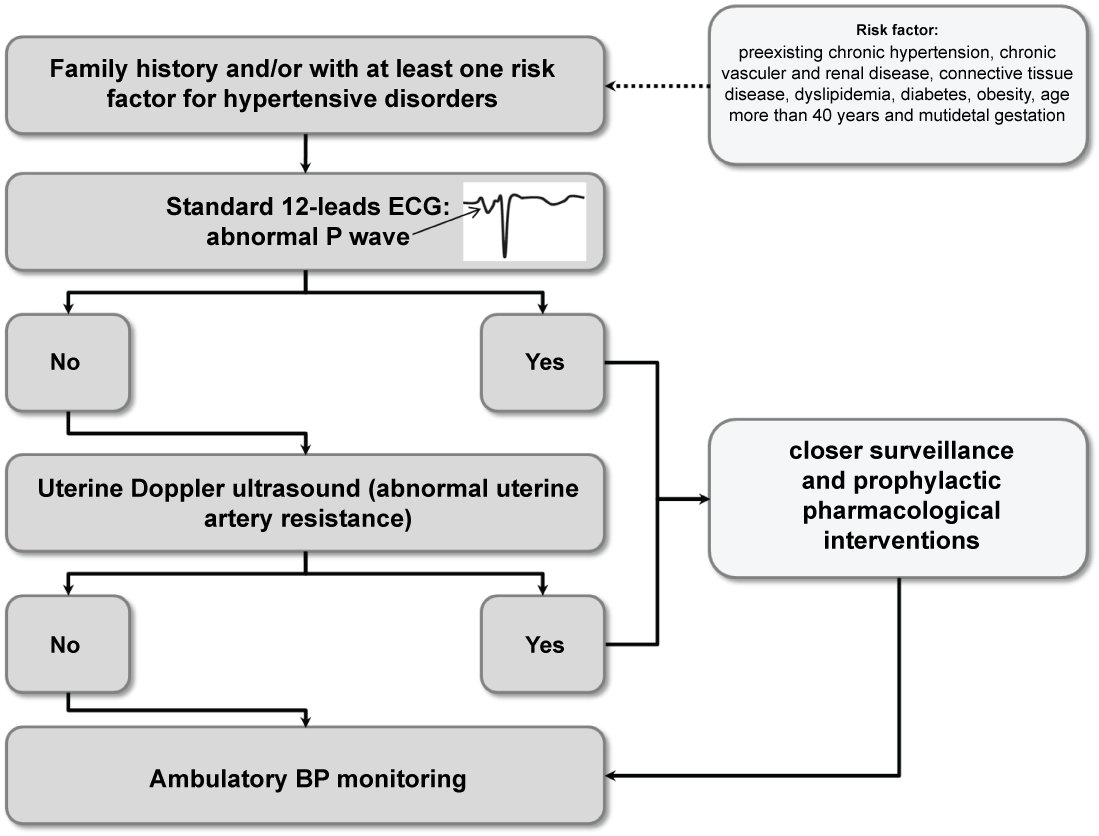 Figure 3: Algorithm for the risk stratification of hypertensive disorders during pregnancy. View Figure 3
Figure 3: Algorithm for the risk stratification of hypertensive disorders during pregnancy. View Figure 3
In the first stage, women with family history of hypertensive disorders during pregnancy or with at least one major risk factor for these conditions (preexisting chronic hypertension, chronic vascular and renal disease, connective tissue disease, dyslipidemia, diabetes, obesity, age more than 40 years and multifetal gestation) should be referred to a specialist to perform a standard ECG in the first trimester of pregnancy. The presence of an abnormal P wave morphology identifies women at high risk for the development of hypertensive disorders during pregnancy. Among those with normal P wave morphology, an uterine Doppler ultrasound with their fetal anatomy scan may be performed to better stratify the risk (specialist referral). Of the women referred for a specialist opinion, ABPM may be also performed during pregnancy to detect masked hypertension and to evaluate pulse pressure and night-time BP.
The primary aim of the proposed new approach to prenatal care is to identify women that would potentially benefit from a closer surveillance and from a prophylactic pharmacological interventions to improve placentation (Figure 3).
Nevertheless, the clinical value of such integrated clinic in which maternal characteristics and history are combined with the results of instrumental techniques to assess the risk for a wide range of hypertensive complications needs to be the subject of well designed prospective studies.
The authors declare that there is no conflict of interests regarding the publication of this paper.