Numerous studies have demonstrated a strong relationship between circulating levels of marinobufagenin (MBG) and salt-sensitivity. Since salt-sensitive hypertensives have increased plasma levels of MBG and are known to be at a higher risk of having cardiovascular events, stroke and increased mortality, we evaluated the possibility of an association between MBG and ischemic stroke. In this pilot study, we determined plasma MBG levels in patients after surviving an ischemic stroke compared to similar age and gender groups of treated hypertensives and normotensive controls.
We measured plasma MBG levels in a total of 40 participants subdivided into three groups: After an ischemic stroke STR (n = 13), participants with a diagnosis of hypertension receiving blood pressure medication HT (n = 14) and normotensive control subjects CTL (n = 13). We used inferential statistics (parametric or non-parametric) and ordered logistic regression models (unadjusted and adjusted) and all statistical analyses were performed using Stata 14.
We did not include a subject from the CTL group because of a diagnosis of glucose-6-phosphate dehydrogenase deficiency and an extreme plasma MBG value of 2,246 pmol/L. Participants' mean age was 60.4 ± 11.5 years; 56% were male. There was no significant difference between study groups (p > 0.05) for gender, age, and body mass index. HbA1c levels were significantly higher in the STR as compared to the CTL (p < 0.05). In the STR group MBG levels were below the normal range (< 200 pmol/L) in three (23%), eight (61%) were in the normal range (200-400 pmol/L), while two (16%) had increased MBG values (> 400 pmol/L). Also, among the STR, the plasma MBG levels did not differ between those receiving and not receiving thrombolytic therapy (p > 0.05). From the 14 HT participants, six (43%) had MBG plasma levels within the normal range, and eight (57%) had high concentrations (> 400 pmol/L). Four (29%) of the treated hypertensives had extreme MBG levels (> 1,000 pmol/L) and normal values of blood pressure.
There was no significant elevation of plasma MBG in survivors 24 h or more after an ischemic stroke. The extreme values of plasma MBG in 29% of the treated hypertensives suggests the presence of salt-sensitivity and a possible side effect of a specific combination of medications. Both of these findings contribute new knowledge to the design of studies to define if there is an MBG molecular mechanism underlying the complex associations among salt-sensitivity, hypertension, and ischemic stroke.
Cardiotonic steroids, Marinobufagenin, Ischemic stroke, Hypertension, Salt-sensitivity
Hypertension continues to be one of the top causes for stroke-related death and disability in the world [1]. Despite numerous epidemiological and clinical studies demonstrating the strong relationship between hypertension and stroke, there is a need for studies that define molecular mechanisms underlying this association [1-3]. Excessive salt consumption contributes to the development and maintenance of hypertension, especially in those individuals that have a higher sensitivity to salt intake. Salt-sensitive hypertensives are also known to be at a higher risk of having cardiovascular events, stroke and increased mortality [4]. Salt-sensitive blood pressure (SSBP) has been shown to be an abnormal phenotype and a risk factor for cardiovascular morbidity and mortality that is independent of blood pressure (BP), with a similar or higher impact on health than increased BP alone [4-6]. Studies that contribute new knowledge to define molecular mechanisms underlying the complex associations among salt-sensitivity, hypertension, and stroke, could lead to the identification of biomarkers for stroke prevention, early diagnosis, and effective intervention.
An increase in salt intake increases the circulating level of cardiotonic steroids (CTS) in both experimental animal and human subjects [7,8]. Endogenous cardiotonic steroids are a group of hormones that have in common a steroid structure and their ability to inhibit or modulate the activity of the Na, K ATPase [9]. They are produced from cholesterol in the adrenal cortex, placenta, and hypothalamus, and are involved in the regulation of blood pressure, heart remodeling, kidney function, oxidative stress, angiogenic and stress signaling, and inflammation [10,11]. CTS also play a role in the pathophysiology of diseases such as uremia, preeclampsia, acute myocardial infarction, terminal renal failure and salt-sensitive hypertension [12-14]. Circulating cardiotonic steroids are usually protein-bound, although a small fraction is in their free form. This free component is the active form, which exclusively binds to antibodies and receptors [15]. The receptor through which CTS acts primarily is the Na, K ATPase, which in addition to its very well-known function as an ion pump, is also a critical signal transducer involved in normal physiology and disease progression [16].
Marinobufagenin (MBG) is an endogenous CTS elevated in diseases associated with alterations in extracellular volume, including preeclampsia and salt-sensitive hypertension [8], essential hypertension and primary aldosteronism [17], heart failure [18] and chronic renal failure [14]. MBG has been shown to modulate in a dose-dependent manner the kidney Na, K ATPase activity and to influence other cellular pathways including selected mouse brain areas [19]. Since salt-sensitive hypertensives have increased levels of MBG and are known to be at a higher risk of having cardiovascular events, stroke and increased mortality, we evaluated the possibility of an association between circulating levels of MBG and ischemic stroke. We hypothesize that high levels of MBG may be associated with the risk of ischemic stroke. We chose to aim at ischemic stroke because it accounts for approximately 80% of all strokes and is intimately related to hypertension. To our knowledge, there are no studies measuring plasma MBG levels in ischemic stroke patients.
In this pilot study, we determined plasma MBG levels in patients after surviving an ischemic stroke compared to similar age and gender groups of treated hypertensives and normotensive controls. Our initial objective was to evaluate the feasibility of using plasma MBG levels as a biomarker for the risk of ischemic stroke associated with salt-sensitivity and hypertension.
This clinical research pilot study was approved by the IRB of the University of Puerto Rico-Medical Sciences Campus (UPR-MSC) (Protocol Number A8960113) and National Institutes of Health (NIH) #11947 (2008-003). All participants completed an informed consent on a voluntary basis. The study included the following groups of participants: 1) Ischemic stroke survivors (STR); 2) Medically diagnosed hypertensives receiving blood pressure medication(s) (HT); 3) Unmedicated subjects without a history of hypertension and with resting systolic blood pressure ≤ 139 mmHg and resting diastolic blood pressure ≤ 89 mmHg (CTL). Participants included in the STR group were patients arriving at the Puerto Rico Medical Center Emergency Room (ER) that were transferred to the Stroke Unit with a diagnosis of ischemic stroke during June 2014 to June 2015 and were able to consent to participate (n = 13). Those in the HT (n = 14) and CTL (n = 13) groups were invited to join the study at the Puerto Rico Clinical and Translational Research Consortium (PRCTRC) facilities at the UPR-MSC.
The exclusion criteria for all subjects was: age less than 21 years, treatment with digoxin (Lanoxin) or digitalis derivatives and K+ sparing diuretics; creatinine levels higher than 1.4 mg/dL, cardio-respiratory arrest or mechanical ventilation, sepsis of any etiology, hemorrhagic stroke, and previous history of ischemic stroke or hepatitis. Since diabetes mellitus is a modifiable risk factor commonly found in ischemic stroke patients [1] and is associated with increased levels of MBG in humans [20], we decided to evaluate STR participants with diabetes. Therefore, diabetes was not considered an exclusion criterion for CTL nor HT participants.
Demographic data, medical history (e.g., diabetes, myocardial ischemia, congestive heart failure, and ventricular arrhythmia) and clinical data including weight and height, blood pressure measurements (systolic and diastolic), pulse rate, and laboratory results were obtained from all participants in this study. Data from cardiac function tests was not included [18]. The Stroke Unit Coordinator obtained the demographic, medical and clinical data for the STR group from the medical records taken by the ER nurse upon admission to the hospital. During their stay at the Stroke Unit, we obtained a sample of heparinized blood for MBG analysis. The sample was transferred within an hour of the blood collection to the PRCTRC laboratory; the whole blood was centrifuged for 15 min at 500-600 g at 4 ℃ and the plasma collected free of the white cells layer; and separated in plasma aliquots. Aliquots in cryo-vials were kept frozen at -80 ℃ until ready for analysis.
A trained clinical coordinator at the PRCTRC, interviewed each participant in the HT and CTL groups to gather data regarding demographics and medical history. Furthermore, a nurse at the PRCTRC took additional measurements (e.g., blood pressure, weight, height, and pulse rate) and drew two blood samples (heparin and whole blood). Both samples were transferred within an hour of collection to the PRCTRC laboratory for appropriate centrifugation. The plasma samples for MBG measurement were separated in aliquots as described above and maintained frozen at -80 ℃ until ready for analysis. HT and CTL serum samples were kept at 4 ℃; the following tests were performed at the PRCTRC laboratory within 24 hours: lipid profile, complete metabolic panel (CMP) and HbA1c.
MBG was isolated from plasma samples using C18 SepPak cartridges (Waters Inc, Cambridge, MA). Unbound MBG levels were measured using a dissociation-enhanced lanthanide fluoroimmunoassay based on a murine monoclonal antibody (anti-MBG 4G4) by an established fluoroimmunoassay protocol [8,14,18]. Details of the method and data on the cross reactivity of the MBG antibody have been reported previously [8].
The MBG assay is a competitive immuno-assay between the immobilized MBG and the MBG presented in the extracted plasma samples. Briefly, a BSA conjugated MBG (MBG-BSA) was fixed to the 96 well immunoassay plate overnight. On the next day, the 96 well plate was washed then blocked with 3% skim milk. The primary anti-MBG antibody 4G4 mb and the extracted plasma samples (or MBG standards) were then loaded onto the MBG-BSA covered plate and incubated for one hour at room temperature. After the primary antibody incubation, the plate was washed twice and incubated with anti-mouse europium tagged secondary antibody (Perkin Elmer, Waltham, Massachusetts, USA) at room temperature for one hour. The plate was washed and then incubated with DELFIA enhancement solution (Perkin Elmer, Waltham, Massachusetts, USA) for 5 min. The time resolved fluorescent signal was read with a Perkin Elmer Enspire plate reader (Perkin Elmer, Waltham, Massachusetts, USA) and the MBG concentration were calculated in GraphPad Prism 7 via non-linear regression.
The statistics were selected taking into consideration the limitation of a small sample size, which may affect the power of our study. Descriptive statistics, including median and interquartile range (IQR), were used to characterize our sample. Chi-squared test or Fisher's Exact test was performed, as appropriate, to determine association between study groups (i.e., STR, HT, and CTL), demographic data and MBG levels (i.e., concentration range as low [< 200 pmol/L], normal [200-400 pmol/L], and high [> 400 pmol/L]). Differences in metabolic, lipids, and blood pressure measurements between study groups were assessed using the one-way ANOVA or Kruskal-Wallis test, as appropriate; significant results in these tests were further analyzed performing ANOVA post-hoc test or Dunn's test using a Bonferroni correction. An ordinal logistic regression was performed to estimate the magnitude of association between MBG levels and the study groups; the proportional odds assumption was assessed using the Brant test. In addition, the STR group data underwent a sub-analysis using Mann-Whitney test in order to compare levels of MBG in plasma between those receiving and not receiving thrombolytic therapy (tPA). A linear regression was performed to determine an association between time span (last date/time known to be well and blood drawn for MBG determination) and MBG levels among patients with stroke. All statistical analyses were performed using Stata v. 14 (College Station, Texas 77845 USA).
We measured plasma MBG concentrations in three different groups of participants: STR, patients after an ischemic stroke (n = 13); HT, participants with a diagnosis of hypertension receiving blood pressure medication (n = 14); and CTL, normotensive control subjects (n = 13). One participant from the CTL group was not included due to a diagnosis of glucose-6-phosphate dehydrogenase deficiency and a plasma MBG value of 2,246 pmol/L. Samples with extremely high MBG values, as well as other plasma samples with normal and intermediate MBG values were chosen at random to replicate the analysis. In two different aliquots, the MBG values obtained were similar.
Table 1 summarizes the characteristics of the study groups. Slightly over 56% of the total study sample were male. Participants' mean age and mean BMI were 60.4 ± 11.5 years and 30.7 ± 5.8 kg/m2, respectively. We found no significant differences between study groups (p > 0.05) for gender, age, BMI, pulse rate, systolic blood pressure and pulse pressure. Diastolic blood pressure levels (mmHg) were higher among participants in the STR group as compared to those in the CTL group (85.7 ± 14.0 vs. 69.5 ± 11.0; p = 0.007).
Table 1: Characteristics of the study groups. View Table 1
Table 2 presents the clinical laboratory measurements with significant differences. Median values of HbA1c (6.1% [IQR = 0.6] vs. 5.5% [IQR = 0.4]; p = 0.005) were significantly higher for the STR group than for CTL group. Consonant with these findings, we found that diabetes mellitus was significantly present in STR patients when compared to the HT and CTL groups (69.2% vs. 28.6% vs. 8.3%, respectively; p < 0.01). Similarly, nine (69%) of the STR patients had hypertension, and seven (54%) had both hypertension and diabetes mellitus. We found no significant differences (p > 0.05) between study groups for any of the following laboratory values: BUN, creatinine, total protein, sodium, potassium, bilirubin, the enzymes AST, ALT, ALP, and lipid profile values: total cholesterol, high-density lipoprotein cholesterol [HDL], low-density lipoprotein cholesterol [LDL] and triglycerides.
Table 2: Clinical laboratory measurements of the study groups. View Table 2
Figure 1 shows the distribution of plasma MBG levels in the three groups. The median of MBG levels of the overall sample (n = 39) in our study was 350.5 pmol/L (IQR = 202.5). The mean plasma MBG level in the CTL group was 351 pmol/L ranging from 217 to 509 pmol/L. The median values of plasma MBG showed statistical difference between CTL and HT groups (326.1 pmol/L [IQR = 133.1] vs. 488.5 pmol/L [IQR = 668.3]; p < 0.01); as well as between STR and HT groups (292.2 pmol/L [IQR = 618.3] vs. 488.5 pmol/L [IQR = 668.3]; p < 0.001). In eleven of the 13 STR we did not find elevated levels of MBG and nine of these eleven (82%) had diagnosed hypertension, of which seven had high blood pressure upon arrival to the ER. In this group of eleven stroke survivors, eight (61.5%) had MBG levels within 200-400 pmol/L and three (23%) had values less than 200 pmol/L (118, 136 and 147 pmol/L). In this group of STR, seven had diagnosed diabetes mellitus, but only three had HbA1c values over 6.5% (7.7, 8.3 and 8.9%) with MBG values of 367, 292 and 136 pmol/L respectively. Only two STR had MBG levels higher than 400 pmol/L but less than 700 pmol/L (511 and 618 pmol/L). These two STR with high plasma MBG, had normal BP with no medication (127/83 mmHg and 124/78 mmHg) at the moment of admission to the ER. Only one of these two had a diagnosis of diabetes mellitus (MBG of 511 pmol/L) with a HbA1c of 6.5%.
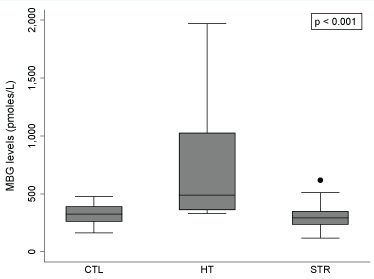 Figure 1: Association between MBG levels and study groups (n = 39).
Figure 1: Association between MBG levels and study groups (n = 39).
MBG: Marinobufagenin; CTL: Control Patients; HT: Hypertensive patients receiving treatment; STR: Stroke patients.
Note: Error bars display the maximum and minimum values (non-outliers) of the data; Outliers were plotted as individual dots (only observed for the STR group). Kruskal-Wallis test was performed to determine an association between MBG levels and the study groups (p < 0.001). Dunn's test using Bonferroni correction was performed for pairwise multiple comparison; MBG levels of the HT group differ from those of CTL (p < 0.01) and STR (p < 0.001) groups, respectively.
View Figure 1
We found a high variation in the MBG values in the HT group. The HT group consisted of 14 diagnosed hypertensives receiving current medical treatment for their high blood pressure. Except for one participant, all had blood pressures within the normal limits of resting systolic blood pressure ≤ 139 mmHg and resting diastolic blood pressure ≤ 89 mmHg. The participant with high BP (142/100 mmHg) was not diabetic and the MBG value was normal (367 pmol/L). Six (42.8%) HT had MBG plasma levels within the normal range (200-400 pmol/L) and four (28.6%) had levels > 400 pmol/L (484, 493, 515 and 747 pmol/L). Of these ten HT participants, only two had diabetes mellitus (MBG levels 391 and 484 pmol/L). Four (28.6%) of the HT participants had extremely high MBG levels > 1,000 pmol/L (1028, 1143, 1208 and 1969 pmol/L). Of these group with extreme MBG levels, only one (1,143 pmol/L) had uncontrolled diabetes mellitus with a HbA1c of 9.1% with medication.
Figure 2 shows no apparent difference in the levels of plasma MBG between the STR group (n = 9) that received thrombolytic therapy (tPA) and those that did not (n = 4). We observed an increase of 1.58 pmol/L (95% CI:-0.80, 3.97) in MBG concentration with increases in one hour in the time span since last time known that the patient was well and the blood sampling for MBG determination (Figure 3).
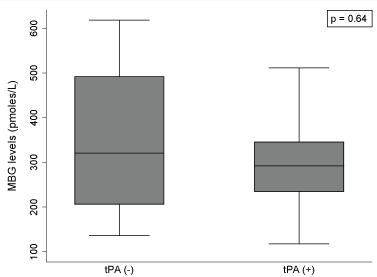 Figure 2: Association between thrombolytic therapy and MBG levels among patients with stroke (n = 13).
Figure 2: Association between thrombolytic therapy and MBG levels among patients with stroke (n = 13).
MBG: Marinobufagenin; tPA (-): Stroke patients not receiving thrombolytic therapy; tPA (+): Stroke patients receiving thrombolytic therapy.
Note: Mann-Whitney test was performed to assess association between tPA and MBG levels among patients with stroke.
View Figure 2
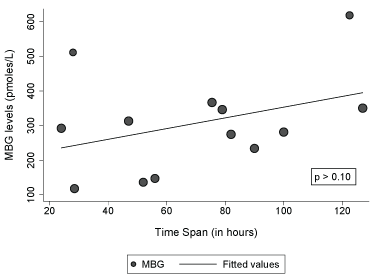 Figure 3: Association between time span (last date/time known to be well and blood draw for MBG determination) and MBG levels among patients with stroke (n = 13).
Figure 3: Association between time span (last date/time known to be well and blood draw for MBG determination) and MBG levels among patients with stroke (n = 13).
Note: Dot sizes represent the final weights assigned to each observation in the model.
View Figure 3
Table 3 shows the distribution of MBG levels, blood pressure and HbA1c values organized by type or group of blood pressure medications taken by the HT group. As shown in Table 3, we found that three of the four HT participants with the highest values of plasma MBG were using a combination of drugs that included an angiotensin II receptor blocker and a calcium channel blocker.
Table 3: Plasma MBG levels, Blood Pressure and HbA1c values for the treated hypertensives (n = 14) organized by blood pressure medications. View Table 3
Marinobufagenin (MBG) is one of the endogenous cardiotonic steroid (CTS) hormones found in the circulation of humans and animals. Although the definitive physiological and pathophysiological roles of MBG are still under extensive evaluation, plasma MBG levels have been found mostly associated with conditions of volume expansion and salt-sensitivity [21]. The mean plasma MBG level in our normotensive control group was 351 pmol/L ranging from 217 to 509 pmol/L. Taking into consideration that our control subjects were on an unrestricted salt diet, the MBG range observed for the controls was consonant with values obtained in normotensive subjects on a high salt diet [22,23].
Nine (64%) of the treated hypertensives had levels of MBG within the range observed for our normotensive control group (329 to 515 pmol/L). Possible explanations for the normal values of MBG found in these treated hypertensive subjects may include: hypertensive treatment reduced the MBG levels, HT participants were not salt-sensitive, or the effect of the hypertensive therapy on the levels of MBG may depend on length and type of treatment. One HT had an intermediate MBG level of 747 pmol/L with normal blood pressure and HbA1c of 5.2%. Four HT subjects showed extreme MBG values (over 1,000 pmol/L). These MBG values are higher than those found in other published studies using the same immunoassay methodology in severe cardiovascular conditions such as primary aldosteronism [17] and heart failure [18]. To our knowledge, values higher than 1,000 pmol/L have been observed in patients with chronic kidney disease [14]. These four subjects indicated in their interviews that they had no kidney disease, which was consonant with their clinical laboratory results and medical history. After a revision of the hypertensive medications taken by these subjects with MBG levels over 1,000 pmol/L, we noticed that three out of four were using the same combination of two drugs to achieve their blood pressure goals: a calcium channel blocker and an angiotensin II receptor blocker. The fourth one had high levels of HbA1c (9.1%) indicating uncontrolled diabetes mellitus. These findings suggest that increased salt-sensitivity, the specific combination of medications or the presence of uncontrolled diabetes may account for higher levels of MBG. The discovery of extreme plasma MBG levels in hypertensive patients on multiple blood pressure medications merits further study.
Salt-sensitive blood pressure has been shown to be a risk factor for cardiovascular morbidity and mortality with a similar or higher impact on health than increased BP alone [4-6]. Using the Dahl salt-sensitive (DS) rat as a model of essential hypertension, we previously reported that total CTS (digoxin-like immunoreactive factor, DLIF) was the same independent of the blood pressure response to high salt and only the free, unbound circulating DLIF increased in those DS rats that had a higher sensitivity to salt intake [7]. This variability in blood pressure response to high salt diet and unevenness in circulating of unbound MBG levels is also found in salt-sensitive subjects with sex-specific [23] and race-related associations [24]. Considering that salt-sensitive hypertensives are known to be: At a higher risk of having cardiovascular events such as stroke, that hypertension is one of the top causes for stroke-related death, and that diabetes mellitus is a risk factor for cardiovascular disease, we evaluated the possibility of finding high levels of MBG associated with having an ischemic stroke.
In this first pilot study evaluating MBG levels after an ischemic stroke, we found that plasma MBG values after 24 hours or more following the onset of the stroke were not increased and were lower or similar to the normotensive controls and significantly lower than the treated hypertensives. Although these findings are in contrast to our initial hypothesis of high MBG values associated with ischemic stroke, it is of importance to note that our results are not indicative of the levels of MBG before or close to the ischemic stroke event. When hypertensive patients have an ischemic stroke, their high values of blood pressure tend to spontaneously subside within 90 min after the stroke and over the following hours and days [25]. In our study, seven of the 13 (54%) ischemic stroke patients arrived at the ER with high blood pressure. Nevertheless, a limitation of the design of this pilot study is that we do not have the blood pressure measurement at the time we obtained the blood sample for MBG. Considering studies showing that MBG has different concentration-dependent effects on the Na, K ATPase ranging from pumping activation to inhibition [26], our results suggest that the low to normal MBG levels observed after 24 hours or more after the ischemic stroke may be the result of a compensatory mechanism. Recent studies support the concept of a protective modulatory role for MBG after an ischemic stroke. For example, experimental ischemia using hippocampal slice cultures found that MBG at low doses (100 pM) significantly increased Na, K ATPase activity protecting against ischemia, while MBG (1000 pM) significantly decreased Na, K ATPase activity [26]. The range of values of MBG that we observed after 24 hours or more of the ischemic stroke in 61.5% of the stroke survivors was within 200-400 pmol/L, and 23% of them had values less than 200 pmol/L (118, 136 and 147 pmol/L).
Preeclampsia is a syndrome related to pregnancy that consists of the development of hypertension and proteinuria accompanied by increments of blood and plasma volumes. There is evidence that women who have both preeclampsia and gestational hypertension are at a higher risk of developing diabetes later in their lives, even in the absence of gestational diabetes mellitus [27]. There is also evidence linking the time course of the pathophysiological changes occurring in preeclampsia with increases in MBG levels [28,29] while other studies found an association between increased levels of MBG with the diagnosis of diabetes mellitus [20]. All these findings suggest that increases in circulating levels of MBG are related to both the development of hypertension and diabetes mellitus, two conditions that are known to increase the risk of cardiovascular disease, including stroke. Consonant with the increased risk of stroke, we found that hypertension and diabetes mellitus were medical conditions diagnosed in nine of the 13 (69%) ischemic stroke patients and co-occurred in seven (54%). The odds of showing MBG levels higher than 400 pmol/L were lower for the STR group as compared to the CTL and HT groups. After controlling for age, sex and HbA1c levels, the odds of higher levels of MBG versus normal/lower MBG levels in plasma of the STR group were 0.37 (95% CI: 0.05-2.46) and 0.10 (95% CI: 0.02-0.62) as compared to the CTL and HT groups, respectively. We discarded the possibility that pulse pressure might be a negative stimulus for the production of MBG since values of pulse pressure did not differ between the three groups. These results point out the need to measure the levels of MBG closer to the ischemic stroke event and at different intervals between the arrival to the ER, transfer and stay at the Stroke Unit.
Since the patients evaluated in this pilot study were ischemic stroke survivors, we hypothesize that the low to normal levels of MBG after 24 hours or more can be a protective mechanism as a component of the endogenous modulatory and regulatory roles proposed for marinobufagenin [11,30]. Furthermore, MBG has been shown to regulate the permeability and gene expression of human brain microvascular endothelial cells in culture [31], increasing the interest in evaluating the possible role of MBG in cerebrovascular and other related vascular disease conditions. Recent studies have investigated the roles of MBG in angiogenic and stress signaling [32], modulation of oxidative stress [33] and neuroinflammation [11]. These findings support the earlier hypothesis that MBG activates endothelial NADPH oxidase [34]. The interaction of MBG at different concentrations with alpha 1 isoforms of the Na, K ATPase, its activation of the non-receptor tyrosine kinase Src by a sodium pump-evoked signal transduction and the activation of oxidant-sensitive enzymes also support the concept that MBG can induce endothelial oxidase stress. These findings contribute to the hypothesis that MBG may be a link between salt sensitivity, increased vascular mortality, and cardiovascular disease, including stroke [11,33,34].
This study has some limitations. First, our study had a small sample size which may have affected the power of our results. Second, we did not perform cardiac function tests to determine if the indices of left ventricular diastolic function are affected by high levels of MBG [14]. Another limitation is that we obtained the clinical data for the STR group at admission to the ER and the MBG sample was collected later when the patient was in the Stroke Unit. Also, we obtained the MBG samples at different time intervals after the stroke event. Despite these limitations, this is the first study measuring MBG levels in ischemic stroke survivors compared to age and sex frequency-matched treated hypertensives and controls. We also report the first case in the literature of an extreme value of MBG (> 2,000 pmol/L) in a normotensive control subject with a diagnosis of glucose-6-phosphate dehydrogenase deficiency.
In conclusion, the low to normal levels of plasma marinobufagenin in 85% of the patients after an ischemic stroke supports the concept of a modulatory role for marinobufagenin. The extreme values of marinobufagenin in 29% of the treated hypertensives suggests the presence of salt-sensitivity and a possible side effect of a specific combination of medications. Both of these findings contribute new knowledge for the design of studies to characterize the particular roles of cardiotonic steroid hormones in the pathophysiology of diseases such as salt-sensitive hypertension and ischemic stroke and to define molecular mechanisms underlying the complex associations among salt-sensitivity, hypertension, and stroke.
This study was supported in part by the National Institute on Minority Health and Health Disparities (NIMHD) under the Award numbers S21MD001830 and U54MD007587; and by Intramural Research program, National Institute on Aging, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors gratefully acknowledge the collaboration and advice of Dr. Manuel Martínez-Maldonado, Dr. Olga V. Fedorova and Dr. Alexei Y. Bagrov; The technical support of our clinical study coordinator Mrs. Claribel González-Rodríguez, the collaboration in recruitment of Dr. Sandra Franqui and Dr. Fernando Santiago from the Stroke Unit, Puerto Rico Medical Center.
This study was supported in part by the National Institute on Minority Health and Health Disparities of the National Institutes of Health Award Numbers: U54MD007587 and S21MD001830.