Contrast-induced nephropathy (CIN) is a serious complication in patients with chronic kidney disease with coronary artery disease who undergo percutaneous coronary intervention (PCI), and is associated with higher morbidity and mortality in short and long term. The limiting volume of contrast agent is safest and most reliable strategy to prevent CIN. Here, we report a case of successful primary PCI using only 13 ml of contrast (iodixanol) of mid left anterior descending artery where wire in diagonal branch served as a landmark in a 75-year-old diabetic and hypertensive male who had presented with ST segment elevation anterior wall myocardial infarction and had marked renal dysfunction having serum creatinine of 3.3 mg%. There was no change in basal renal function after the procedure and safely discharged.
Contrast-induced nephropathy, Percutaneous coronary intervention, Acute coronary syndrome, Contrast agent, Left anterior descending artery
Contrast-induced nephropathy (CIN) is associated with increased morbidity and mortality including the need for renal replacement therapy (RRT) which is defined as absolute (≥ 0.5 mg/dL) or relative increase (≥ 25%) of serum creatinine from baseline after 48-72 hours following contrast administration, when alterative explanations have been excluded [1,2]. Peri-procedural hydration and minimum possible volume of contrast are the only established measures to prevent CIN [1,3]. The risk of CIN which is 0.6-2.3% in general pop-ulation and approaching to 20% in high risk patients, and requirement of RRT are the prime factors responsible for underutilization of percutaneous coronary intervention (PCI) in patients with established chronic kidney disease or those with baseline renal impairment [4].
A 75-year-old diabetic and hypertensive male presented with chest heaviness with sweating of 6 hours duration. An electrocardiogram showed marked ST elevation in V1-V6. Routine haemogram and biochemistry was normal except deranged renal function with serum creatinine of 3.3 mg%. His body weight was 68 kg. Echocardiography revealed mild concentric left ventricular hypertrophy, regional wall motion defect in left anterior descending territory (LAD), and impaired systolic function with an ejection fraction of 40%. His coronary angiogram was done through transfemoral approach after proper consent revealing critical lesion with 90% stenosis in mid left anterior descending (LAD) artery just after large diagonal branch, normal left circumflex territory, and right coronary artery (Figure 1 and Figure 2). The coronary angiogram was completed using 9 ml of contrast. The contrast used was iodixanol which is an iso-osmolar contrast agent. Primary PCI was planned subsequently. Intravenous heparin (100 U/Kg) was administered. Based on all the baseline factors, it was a high risk PCI carrying a profound risk of CIN. To minimize the risk of CIN, intravenous normal saline infusion was started. Prior to PCI, initial angiogram with same angle of projection was uploaded to the monitor for PCI guidance. Left main artery was engaged with 6F extra backup guiding catheter (EBU; Medtronic, USA) which was confirmed by entry of the runthrough wire (Terumo, Japan) into the coronary artery. An exaggerated curve was created to facilitate the wiring of LAD. Additional runthrough wire was placed in first diagonal branch (D1) based on the previous angiogram (Figure 3). The side branch wire served as an important landmark to guide PCI and to protect the side branches as well. The balloon was positioned just after D1 which was used as a landmark for proximal end for stent placement. Lesion was gradually predilated with 2 × 10 and 2.5 × 10 pantera leo semi compliant balloons (Biotronik, Germany). 3 × 23 mm Endeavour Resolute (Zotarolimus eluting stent; Medtronic, USA) stent was positioned across the lesion keeping the proximal end at the crossing of LAD and D1 wire which was serving as the origin of D1 (Figure 3). It was deployed at 13 atm pressure (Figure 4). As it was not fully expanded, it was serially post dilated by 3 × 10 and 3.5 × 10 non-compliant Minitrak balloon (Abott, USA) at 24 atm pressure. The stent apposition was checked under stent boost. After confirmation of stent optimization with stent boost, final angiography with 4 ml contrast injection revealed successful results (Figure 5). Therefore, PCI was completed using 13 ml of contrast. He was clinically stable for 2 days without CIN (Serum creatinine 3.5 mg/dL). She was discharged 2 days after PCI with appropriate drugs and has been uneventful.
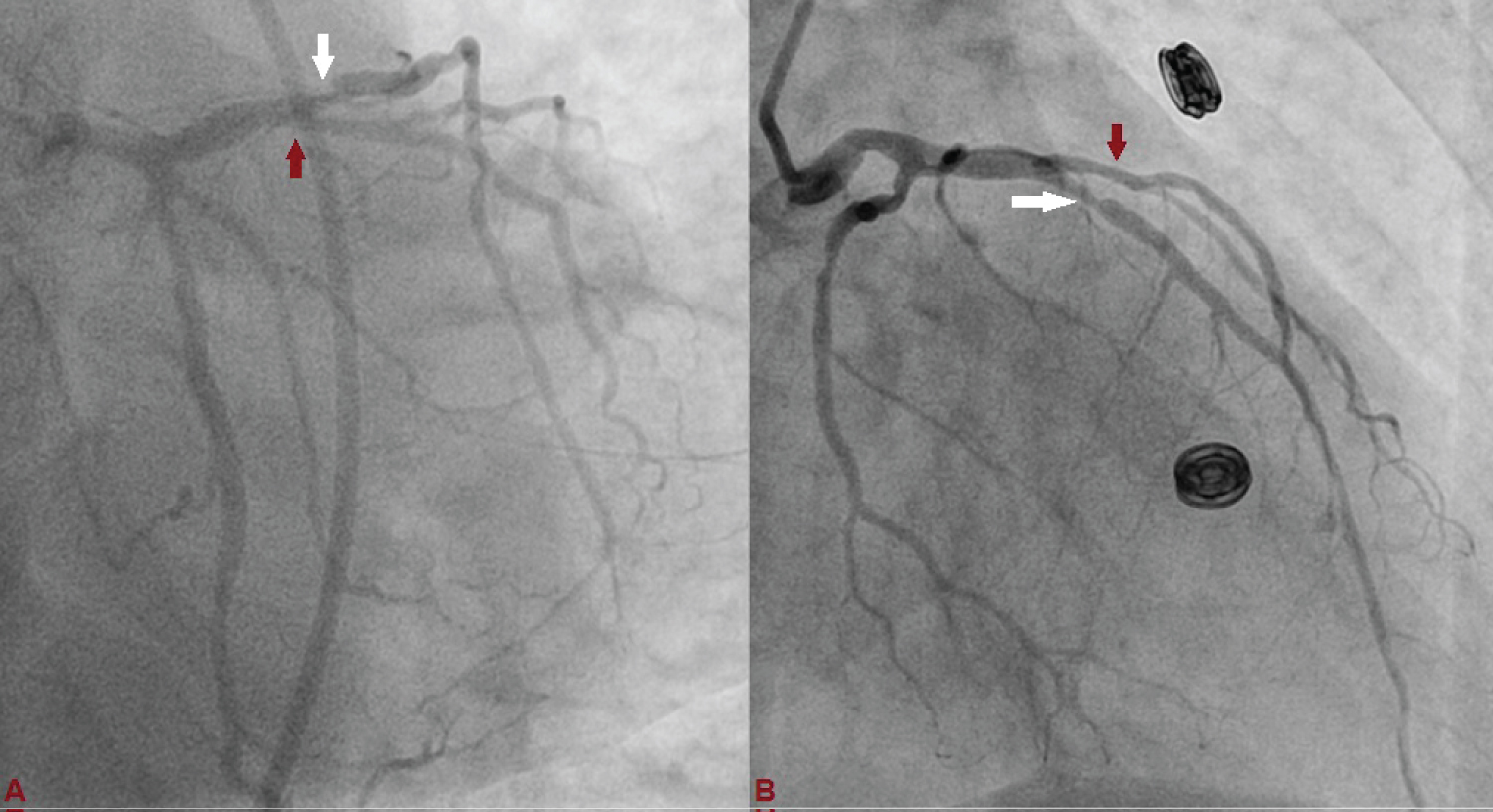 Figure 1: Left anterior descending (LAD) artery revealing critical lesion with 90% stenosis in mid just after large diagonal branch (D1), and normal left circumflex artery. (A: Antero-posterior view with caudal angulation; B: Antero-posterior view with cranial angulation).
View Figure 1
Figure 1: Left anterior descending (LAD) artery revealing critical lesion with 90% stenosis in mid just after large diagonal branch (D1), and normal left circumflex artery. (A: Antero-posterior view with caudal angulation; B: Antero-posterior view with cranial angulation).
View Figure 1
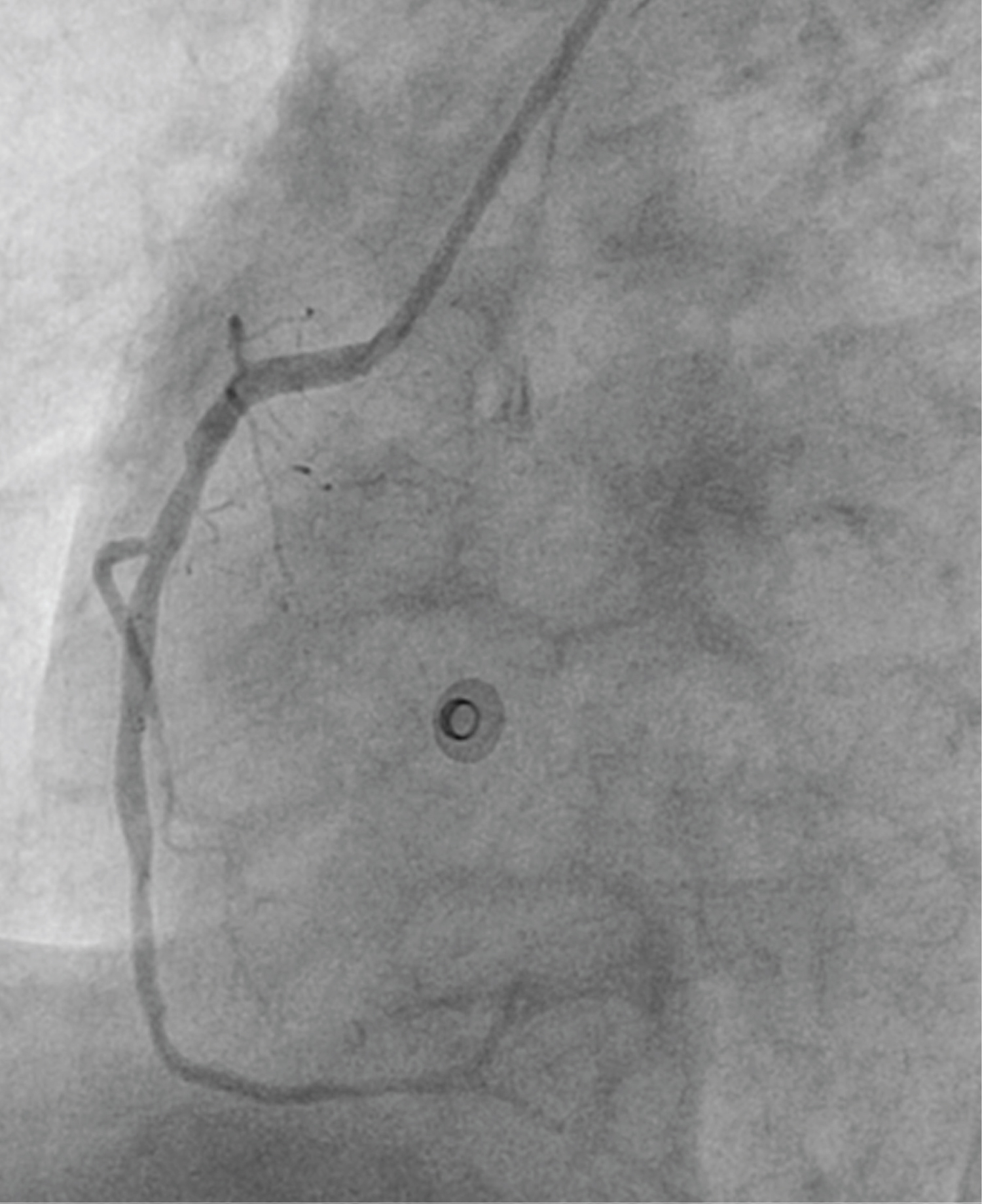 Figure 2: Left anterior oblique view showing normal flowing right coronary artery.
View Figure 2
Figure 2: Left anterior oblique view showing normal flowing right coronary artery.
View Figure 2
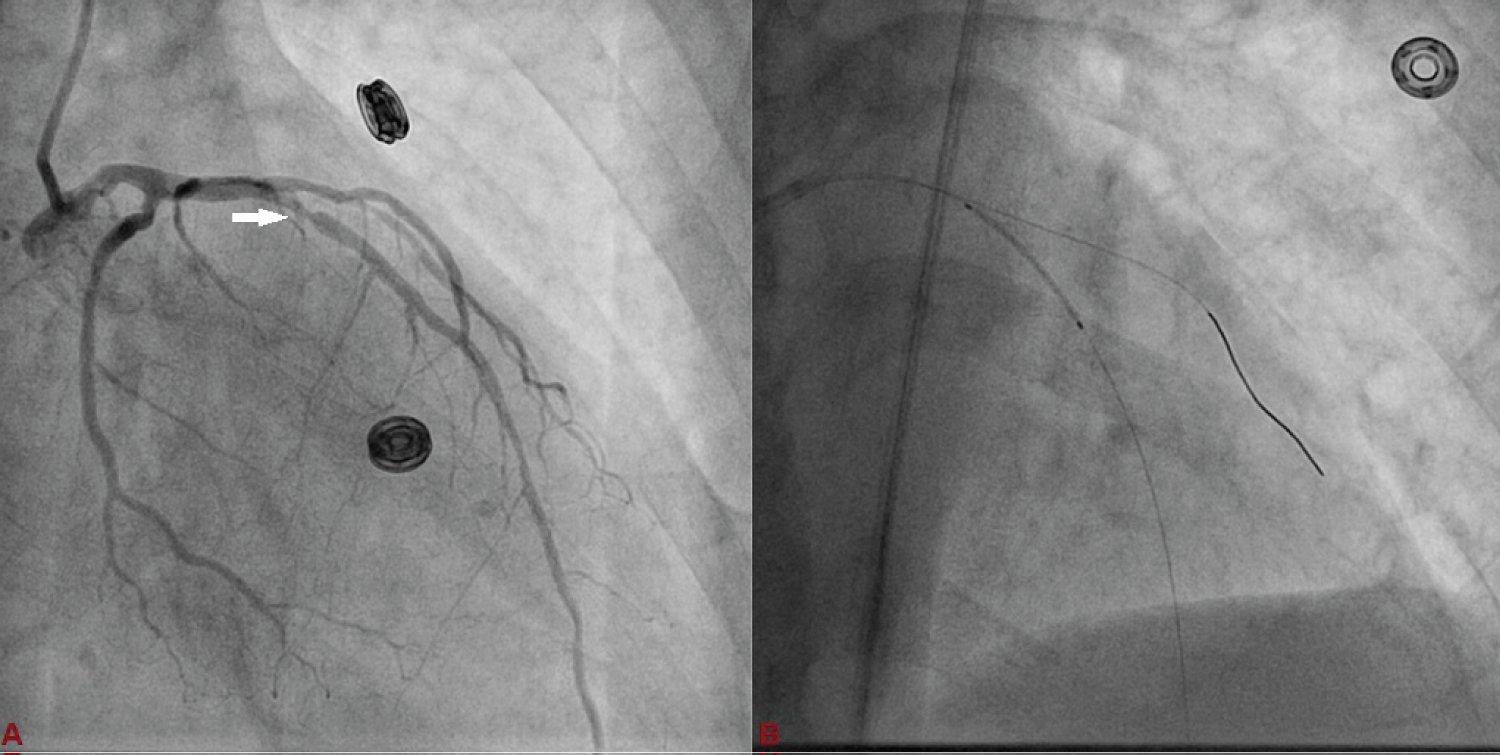 Figure 3: A) Basal angiographic view of left system used as anatomical reference to mark position of LAD and D1; B) 3 × 23 mm Endeavour Resolute stent was positioned across the lesion keeping the proximal end at the crossing of LAD and D1 wires which was serving as the origin of D1.
View Figure 3
Figure 3: A) Basal angiographic view of left system used as anatomical reference to mark position of LAD and D1; B) 3 × 23 mm Endeavour Resolute stent was positioned across the lesion keeping the proximal end at the crossing of LAD and D1 wires which was serving as the origin of D1.
View Figure 3
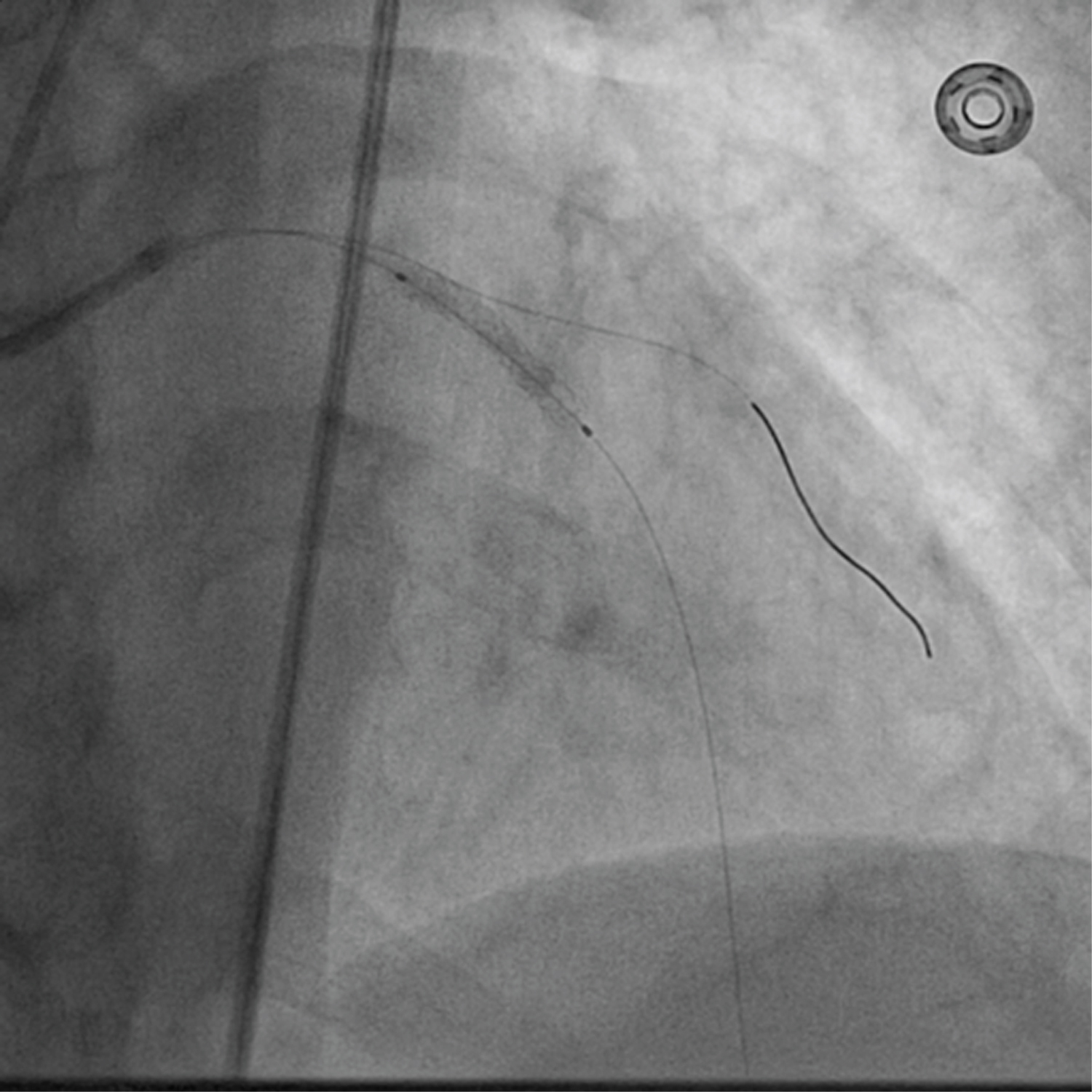 Figure 4: Stent was deployed at 13 atm pressure.
View Figure 4
Figure 4: Stent was deployed at 13 atm pressure.
View Figure 4
 Figure 5: Final angiography with 4 ml contrast injection revealed successful PCI of LAD with TIMI III flow.
View Figure 5
Figure 5: Final angiography with 4 ml contrast injection revealed successful PCI of LAD with TIMI III flow.
View Figure 5
CKD and CAD share several risk factors, and therefore patients with CKD have a higher atherosclerotic burden [5]. Presence of advanced atherosclerosis is also major risk factors for CIN, thus complicating PCI in patients with CKD and thereby making revascularization an under-utilized option in this subset of patients. These patients who undergo revascularization have survival advantage over medical therapy alone based on observational studies although randomized trials are lacking [5]. However, concern regarding CIN and requirement of RRT offsets the performance of revascularization in patients with CKD. Pre-existent renal disease is the strongest independent predictor for development of CIN and the requirement for RRT, which develop in 27 and 4% of patients with severe CKD, respectively. The 2014 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines on myocardial revascularization advocate ad hoc rather than staged PCI in patients with CKD and extensive atherosclerosis, if substantial contrast use in one single invasive procedure can be avoided, to minimize the risk of acute kidney injury secondary to atheroembolism [6]. Despite many proposed modality to treat CIN, prevention is the best strategy to improve the outcome after PCI as in-hospital, 1-year, and 5-year mortality rate are significantly higher in patients who develops CIN compared to those who don’t (1.4% vs. 22%), (3.7% vs. 12.1%), and (14.5% vs. 44.6%) respectively [7]. Contrast volume as minimum as possible is the safest and most reliable strategy which can be done in several ways including pre-interventional imaging to plan procedure, images of target vessel anatomy as reference, use of diluted contrast, iso-osmolar contrast, imaging modalities such as intravascular ultrasound (IVUS), and hybrid imaging [7]. IVUS guided PCI has 3-fold lower usage of contrast compared to angiography-only approach, thus making it an attractive alternative imaging tool to guide PCI [7]. The drawback is that it is costlier, requires operator expertise, a learning curve, and accurate image interpretation which make it potentially not very attractive. Furthermore fluoroscopy time, the number of cine runs, or radiation dose may be higher for less experienced operators though it was not reported in study by Mariani J Jr, et al. [8]. IVUS carries an advantage that it gives valuable information on cross-sectional anatomy, appropriate landing zone, diameter and length of stent, and accurate apposition which therefore minimizes the complications. Although in our case, lesion was type A, considering the baseline profile of the patient, risk of CIN was very high which made it an extremely high risk procedure. In our case, minimal dosage of contrast at the end of the procedure was used to confirm any injuries in the form of dissection, apposition, and status of side branch. Therefore, it was demonstrated that ultra-low contrast volume reduces the rate of CIN in patients with chronic renal disease who undergo PCI, which can be done with limited resources where IVUS is not available. The lack of worsening renal function following PCI in our case suggests that CIN is the major cause of acute nephropathy following revascularization, and staged procedure may be considered as an alternative in extremely high-risk group of patients.
None.
Disclosure of funding received for this work from any of the following organizations: National Institutes of Health (NIH); Welcome Trust; and other(s) - None.
All authors made equal contribution.