Our discovery of "calcium paradox" phenomenon due to interaction of Ca2+/cAMP intracellular signalling pathways (Ca2+/cAMP interaction) may provide new insights for the treatment of psychiatric disorders, such as depression. This disorder is mainly resulting by reduction of serotonin and catecholamine release in central nervous system. Since 1975, several clinical studies have reported that administration of L-type Ca2+ channel blockers (CCBs) produces reduction in vascular resistance and arterial pressure in hypertensive patients, associated with an increase in plasma noradrenaline levels and tachycardia characterized by sympathetic hyperactivity. During almost four decades, these enigmatic phenomena remained unclear. In 2013, we discovered that this paradoxical sympathetic hyperactivity produced by CCBs is mediated by Ca2+/cAMP interaction. Then, the pharmacological manipulation of this interaction could be a more efficient therapeutic strategy for increasing serotoninergic and monoaminergic neurotransmission in depression.
Depression is a psychiatric disease resulting mainly by dysfunction of monoaminergic neurotransmission in central nervous system [1,2]. Depression is a severe global illness, becoming more and more common each decade. Because of specific symptoms, it is considered as a leading cause of disability all over the world with a high death factor due to suicides. There are many antidepressants used in the therapy, but still more than one-third of patients do not respond to the current therapy [2]. The heterogeneous nature of the illness and its complex, and unclear etiology, may be responsible for treatment difficulties. Next to the main monoaminergic hypothesis of depression, there are also many other approaches connected with the pathophysiology of this disease, including hypothalamic-pituitary-adrenal axis dysregulation, dopaminergic, cholinergic, glutamatergic or gamma-aminobutyric acid (GABA)-ergic neurotransmission [2]. Nevertheless, it can be unambiguously stated that serotonergic, noradrenergic and dopaminergic systems (monoaminergic neurotransmission) are precisely associated with pathogenesis of depression, and should be therefore considered as valuable targets in patients' treatment. In this review, we discuss novel strategies to treat depression, throughout our recent discovery entitled "calcium paradox" phenomenon due to Ca2+/cAMP interaction [1,3,4].
Depression is an incapacitating psychiatric condition that causes a significant problem on individuals and society. There is still a lack of a clear understanding of the neuropathological changes associated with this illness, and the efficacy of antidepressants is still far from the best [5]. Research into antidepressant therapies has derived from observations in human trials and animal models after the first monoaminergic hypothesis emerged (about six decades ago). However, glutamatergic modulators, such as ketamine also have become the forefront of antidepressant exploration, especially for treatment-resistant depression and suicidal ideation [5]. The glutamatergic hypothesis of depression is not novel, however other N-methyl-D-aspartate (NMDA) receptor modulators do not seem to share the rapid and sustained effects of ketamine, suggesting that a unique combination of intracellular targets might be involved in its effect [5,6]. Interestingly, inflammation can impact the glutamatergic system enhancing excitotoxicity and decreasing neuroplasticity. The points of convergence between the inflammatory and glutamatergic hypotheses of depression are not completely established, especially regarding the effects of fast-acting antidepressants [5,6].
Nonetheless, the monoamine hypothesis of depression continues to dominate the field and clinical trials, which postulates that an imbalance in monoaminergic neurotransmission is causally related to the clinical features of depression [6]. Antidepressants influence serotonin whose mainly goal consist at raising serotonin concentrations, thereby increasing serotonergic transmission at the level of the synapse, for example by inhibiting the serotonin transporter. However, the serotonin system is multifaceted. Different serotonin receptor subtypes turn the serotonergic system into a complex neurochemical arrangement that influences diverse neurotransmitters in various brain regions. Classical antidepressants, as well as other psychopharmacological agents have various crucial effects on serotonin receptors. Researchers aim to provide a useful characterization of serotonin receptor subtypes in the treatment of depression. Clarifying the mode of action, and the interplay of serotonin receptors with pharmacological agents should help elucidate antidepressant mechanisms and typical side effects to better understanding. In addition, clinical medicine featured the novel antidepressants vortioxetine, vilazodone and milnacipran/levomilnacipran with regard to their serotonin receptor targets such as the 5-HT1A (5-hydroxytryptamine, 5-HT), 5-HT3 and 5-HT7, which may account for their specific effects on certain symptoms of depression as well as a characteristic side-effect profile [6].
The combination of novel ideas added to improvements on the discoveries may lead to advances in antidepressant research with the promise of finding compounds that are both effective, and fast-acting, including in patients who have tried other therapies with limited success. In conclusion, new insights for more efficient pharmacological treatments of depression are clearly needed.
Several experiments initiated decades ago using catecholaminergic cells originated the concept of stimulus-secretion coupling to elucidate neurotransmitter release and hormone secretion. This concept was initially resulted from the study of cat adrenal gland perfused with acetylcholine executed by Douglas and Rubin in the 1960s [7]. The discovery that increase in the cytosolic Ca2+ concentration ([Ca2+]c) was a basic requirement for exocytosis in adrenal catecholaminergic cells was made by Baker and Knight in 1970's [8]. In addition, some studies showed that cAMP rises transmitter release at many synapses in autonomic nervous system of vertebrate, including sympathetic and parasympathetic ganglion neurons [9]. Although the cellular and molecular mechanisms involved in these synergistic actions of cAMP on the exocytosis of neurotransmitter and hormones remain uncertain, the evidences suggest that this intracellular messenger can participate in fine regulation of exocytosis due to its modulatory action on the intracellular Ca2+ signals.
In fact, the hypothesis for Ca2+/cAMP interaction has been extensively studied in many cells and tissues. Generally, this interaction results in synergistic effects on cell functions [1,3,4,10,11] and occurs at the level of adenylyl cyclases (ACs) or phosphodiesterases (PDEs) (Figure 1).The Ca2+/cAMP interaction has particularly been extensively studied at the Ca2+ channels [e.g.: ryanodine receptors (RyR)] of the endoplasmic reticulum (ER) [1,3,4,10,11]. Phosphorylation of RyR by protein kinase A (PKA), and also inositol trisphosphate receptor (IP3R) at submaximal IP3 concentrations, may increase the open probability of ER Ca2+ stores, amplifying Ca2+-induced Ca2+ release (CICR) mechanism and cellular responses [1,3,4] (Figure 1). Recent evidences suggest that Ca2+/cAMP interaction participates of exocytosis regulation in neurons and neuroendocrine cells [1,3,4]. Then, dysfunctions of cellular homeostasis of Ca2+ and/or cAMP in these cells could result in the dysregulation of Ca2+/cAMP interaction and exocytotic response, or could be a novel therapeutic target for medicines (Figure 1).
 Figure 1: Role of Ca2+/cAMP interaction in neurotransmitter release, including monoamines from central system nervous. Cellular homeostasis of Ca2+ and/or cAMP in these cells could result in the dysregulation of Ca2+/cAMP interaction and exocytotic response of monoamines, or could be a novel therapeutic target for medicines, according to our previous studies [1,3,4]. cAMP can enhance neurotransmitter release, thus producing anti-depressant effect. Cellular responses: in monoaminergic cells.
View Figure 1
Figure 1: Role of Ca2+/cAMP interaction in neurotransmitter release, including monoamines from central system nervous. Cellular homeostasis of Ca2+ and/or cAMP in these cells could result in the dysregulation of Ca2+/cAMP interaction and exocytotic response of monoamines, or could be a novel therapeutic target for medicines, according to our previous studies [1,3,4]. cAMP can enhance neurotransmitter release, thus producing anti-depressant effect. Cellular responses: in monoaminergic cells.
View Figure 1
Since 1975, several clinical studies have been reporting that acute and chronic administration of L-type CCBs, such as nifedipine and verapamil, produces reduction in peripheral vascular resistance and arterial pressure associated with an increase in plasma noradrenaline levels and heart rate, typical signals of sympathetic hyperactivity [12]. However, the cellular and molecular mechanisms involved in this apparent sympathomimetic effect of the L-type CCBs remained unclear for decades. In addition, experimental studies using isolated tissues richly innervated by sympathetic nerves showed that neurogenic responses were completely inhibited by L-type CCBs in high concentrations (> 1 μmol/L), but paradoxically potentiated in concentrations below 1 μmol/L [13-15]. During almost four decades, these enigmatic phenomena named by us as "calcium paradox" remained unclear. In 2013, we discovered that this paradoxical increase in sympathetic activity produced by L-type CCBs is due to Ca2+/cAMP interaction [1,3,4]. Then, the pharmacological manipulation of the Ca2+/cAMP interaction produced by combination of the L-type CCBs used in the antihypertensive therapy, and cAMP accumulating compounds used in the anti-depressive therapy such as rolipram, could represent a potential cardiovascular risk for hypertensive patients due to increase in sympathetic hyperactivity. In contrast, this pharmacological manipulation could be a new therapeutic strategy for increasing neurotransmission in the psychiatric disorders, such as depression.
In addition, several studies have been demonstrating pleiotropic effects of CCBs. CCBs, like nifedipine, genuinely potentiate the effect of tricyclic and atypical antidepressants [16,17]. However, the molecular mechanisms involved in these pleiotropic effects remain under debate. In fact, apart from its classical functions, CCBs are described to have beneficiary roles on the cognitive profile of the aged population and individuals with hypertension, diabetes, Parkinson's disease, and Alzheimer's disease [18-21]. Different mechanisms have been proposed, but the exact mechanisms of antidepressant effects and cognitive improvement are still uncertain.
In contrast to adverse effects produced by combination of L-type CCBs with cAMP accumulating compounds in the cardiovascular diseases, the pharmacological implications of the Ca2+/cAMP interaction produced by this drug combination could be used to enhance neurotransmission [1,3,4].
Recent studies have showed that chronic treatment with rolipram, together with typical antidepressants has been successful in the reduction of depression symptoms due to potentiation of these antidepressants effects [22-24]. Considering our model in which increment of [cAMP]c stimulates Ca2+ release from ER (Figure 1), it may be plausible that the therapeutic use of the PDE inhibitor rolipram (Figure 2) [23,24], in combination with low doses of verapamil to potentiate neurotransmission (Figure 1) in the areas of central nervous system involved in neurological/psychiatric disorders in which neurotransmission is reduced, including depression. This new pharmacological strategy for the treatment of psychiatric disorders could increase the therapeutic efficacy and reduce the adverse effects of the medicines currently used for treating depression. Considering that CCBs genuinely exhibit cognitive-enhancing abilities and reduce the risk of psychiatric disorders like depression [17]; and that the mechanisms involved in these pleiotropic effects are largely unknown. Then, whether Ca2+/cAMP interaction is involved in such effects deserves special attention.
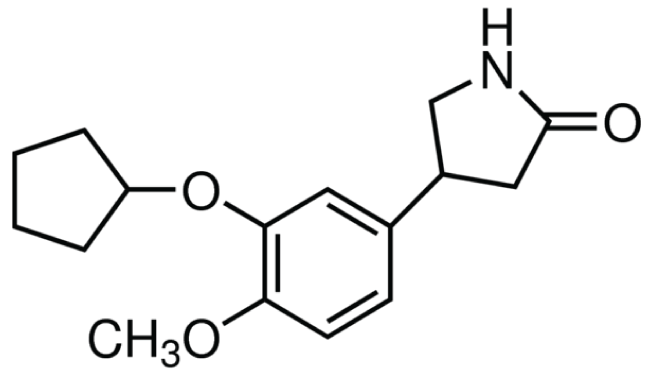 Figure 2: Chemical structure of rolipram: selective cAMP-specific phosphodiesterase (PDE4) inhibitor. Extracted from Sigma-Aldrich.
View Figure 2
Figure 2: Chemical structure of rolipram: selective cAMP-specific phosphodiesterase (PDE4) inhibitor. Extracted from Sigma-Aldrich.
View Figure 2
In addition, considering [Ca2+]c elevation could contribute to both: negatively to neuroprotective effects and positively to exocytosis, it may be plausible the therapeutic use of the PDEs inhibitors [23,24] for antidepressant purposes. Then, pharmacological interference of the Ca2+/cAMP interaction produced by combination of L-type CCBs and cAMP-accumulating compounds could enhance antidepressant response and reduce clinical symptoms of psychiatric disorders. Thus, the association of currently medicines could enhance antidepressant treatments. For example: the association of typical antidepressants with CCBs or rolipram (Figure 2) could dramatically improve typical antidepressant medicines, mainly by reducing their adverse effects and increasing their effectiveness. This new pharmacological strategy could be alternatively used for treatment of the symptoms of psychiatric disorders, including depression.
The diagnosis of psychiatric disorders like depression relies critically on collaborative history of patients. In addition, emerging therapies may supplement clinical assessment in the next years. Although pharmacological therapies have been largely unsuccessful in curing depression, targeting potential risk factors aiming to decrease incidence of this psychiatric disorder is an important public health edge. Finally, novel strategies to treat depression, throughout our recent discovery entitled "calcium paradox" phenomenon due to Ca2+/cAMP interaction, could greatly contribute to enhance therapeutic strategies for increasing neurotransmission. Thus, the association of typical antidepressants with CCBs or rolipram could dramatically improve antidepressant therapies, mainly by reducing adverse effects and improving effectiveness of these typical antidepressants.
Caricati-Neto and Bergantin thank the continued financial support from CAPES, CNPq and FAPESP (Bergantin's Postdoctoral Fellowship FAPESP #2014/10274-3).