Surface-enhanced Raman scattering (SERS) is a surface sensitive method that results in the enhancement of Raman scattering by molecules adsorbed on rough metal surfaces. The enhancement factor can be as much as 107 -1015, which allows the technique to be sensitive enough to detect single molecules. The rapid growth of pharmaceutical industries worldwide demands continuous development of efficient characterization techniques to detect the presence of the molecules at extremely low concentrations. SERS allows fast, sensitive detection of trace levels of key pharma molecules. This review highlights the requirements for SERS and its applications for pharmaceutical characterization.
Nanomaterials, Synthesis, SERS, Drugs
Raman spectroscopy is concerned with radiation scattering from a sample. Scattering occurs when an incident photon interacts with the electric dipole of a molecule. This scattering process can be either elastic or inelastic. Most incident photons are elastically scattered by the molecule (Rayleigh scattering). In Rayleigh scattering, the energy of the incident photons equals the energy of the scattered photons. Raman Scattering is a small fraction of light that is scattered at energies different than that of the incident photons (Raman Effect). The Raman Effect is an inelastic process and was first observed in 1928. Chandrasekhara Venkata Raman awarded Nobel Prize in 1930. Two situations arise with Raman scattering; (i) Scattered photons have a lower energy (Stokes scattering-phonon emitted), (ii) Scattered photons have a higher energy (anti-Stokes scattering-phonon absorbed), Figure 1.
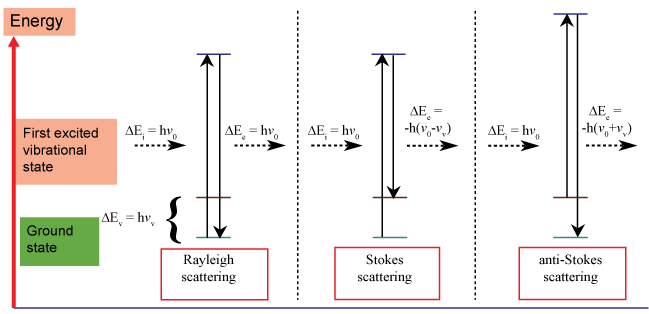 Figure 1: Illustration of some Raman scattering process. View Figure 1
Figure 1: Illustration of some Raman scattering process. View Figure 1
Raman spectroscopy is very useful in drug analysis due to advantages such as ease of use, minimal sample handling, and the significant differences in scattering strength between packaging materials, tablet excipients, and active drug components [1-3]. It can also be used to identify isomers and to determine energy difference between isomers. These advantages, in combination with fiber optics and microscopes, have enabled the use of Raman spectroscopy as a quality control tool in the pharmaceutical industry. One major disadvantage with conventional Raman spectroscopy is the small scattering cross section of many materials.
Since its discovery in 1974, Surface-enhanced Raman scattering (SERS) has been fast developing an analytical method for detection of molecules, particularly in molecule sensing and characterization. SERS is an emerging method for studying the vibrational fingerprints of molecules. The observation of greatly enhanced Raman intensities for molecules after adsorption on substrates such as Ag, Au, and Cu, has been an experimentally and theoretically interesting phenomenon [4]. The SERS effect becomes strong if the frequency of the excitation light is in resonance with the main absorption band of the molecule being illuminated. Due to the high intensity of the Raman signal, SERS method is a highly useful technique in both surface science and analytical [5].
SERS is a form of Raman spectroscopy in which an unusually high Raman scattering cross‐section is achieved when an analyte molecule gets adsorbed to a metallic nano-size surface. SERS involves adsorption of targeted molecules onto the nanosubstrate surfaces. Substrates are of a variety of metals, like silver, gold, or copper with differing morphologies. Generally, gold and silver are most often used as SERS substrates because they are air stable materials. For example, the adsorption of methimazole molecules on silver nanoparticles, Figure 2. The coinage metals of Au, Ag, and Cu are usually used since the resonance condition for these metals lies at common laser frequencies for Raman spectroscopy. In addition, at the resonance frequency, the dielectric function for these metals is minor. The simplistic explanation based on the SERS is that the intensity of the Raman scattering is proportional to the induced dipole of the given molecule. The induced dipole is proportional to the polarizability of the molecule and the magnitude of the incident electric field. The main steps proposed in electromagnetic theory containing (i) An analyte is adsorbed on a surface patterned or roughened so that the chosen excitation frequency will excite a plasmon and create scattering; (ii) Energy with a proper wavelength of excitation, from the plasmon, is transferred to the adsorbed molecules and the Raman process occurs on the molecule; and (iii) Energy is transferred back to the plasmon less the amount transferred to the nuclei and scattered from the surface as wavelength shifted light. These simple steps are the main steps.
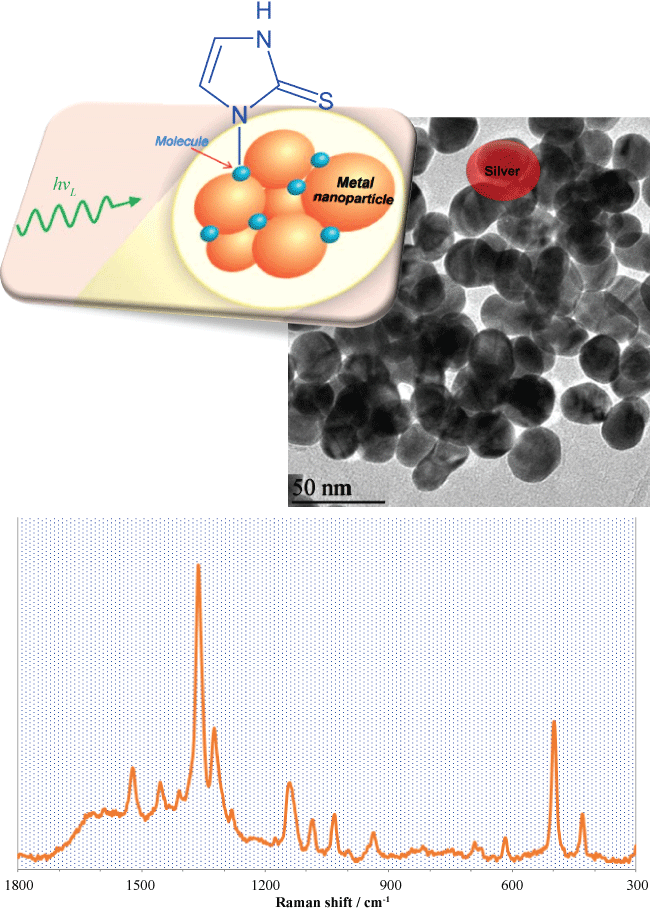 Figure 2: SERS spectra of methimazole on silver nanoparticles with TEM image of silver nanoparticles. View Figure 2
Figure 2: SERS spectra of methimazole on silver nanoparticles with TEM image of silver nanoparticles. View Figure 2
The last decade has seen major advances in the application of SERS and Raman spectroscopy primarily because of the improvements made in Raman instrumentation. The Raman instrumentation consists of lasers, spectrometers, optics and detectors.
• Lasers: The laser excitation frequency is the major determinant of the information content of a Raman spectral measurement. Both continuous and pulsed lasers are used.
• Optics and Filters are used to remove the Rayleigh scattered photons.
• Spectrometers, Detectors: The purpose of the Raman spectrometer is to reject the intense Rayleigh scattered light and to disperse the Raman scattered light into its component frequencies for detection. If the Rayleigh light can enter the spectrograph un attenuated, it will obscure all or part of the much weaker Raman spectrum. The most common and still most versatile Raman spectrometers utilize holographic dispersive gratings and CCD multichannel detectors. These spectrometers are useful from the UV to the near-IR spectral region. Photomultipliers were the standard detectors used until recently. CCD (charge coupled detector) are now more commonly used.
• SERS substrates: commonly used Silver (Ag), gold (Au) and copper (Cu).
• The energy required to generate plasmons matches the light sources typically used in Raman spectroscopy.
There are several parameters and conditions that are to be optimized to obtain enhanced Raman signal and to ensure maximum signal generation and enhancement. These parameters include the selection of excitation source, the features of the substrate, and the ratio of the sample to the substrate. The electromagnetic enhancement is strongest where the particles have the highest curvature; thus, the adsorption of the analyte on the long or narrow axis of an ellipsoid or spheroid effects the magnitude enhancement.
SERS is used to investigate the vibrational properties of adsorbed molecules yielding structural information on the molecule and its local interactions. Uniquely identifies molecules. Enables the detection of individual molecules.
There are many forms of SERS substrates depending on the purpose they are used for different applications [6,7]. SERS was first observed on the roughened surface of electrodes [8,9]. The Raman spectrum of pyridine was enhanced to almost more than million times in SERS on metal colloids [10]. This phenomenon was called SERS and it was realized that the nature of the substrate plays an important role in the enhancement [11]. Nano substrates from metals such as gold, silver, and copper exhibit enhancement of Raman spectra [12]. Figure 3 shows TEM image of silver nanoparticles. Every material has a characteristic plasmon (collective oscillations of electrons) associated with it, which is size dependent. When the mean free path of the electron exceeds the size of the structure; 10 nm to 100 nm, the plasmon is mostly associated with the surface. When a light matching the plasmon frequency of the nanostructure is incident on it, it excites the surface plasmon of the nanosubstrate. This is called surface plasmon resonance [13]. The excited surface plasmon produces an oscillating dipole leading to the generation of the source, producing a local electromagnetic electromagnetic (EM) radiation matching the excitation enhancement very close to the surface (~1 nm of the nanosubstarte). When a molecule is in the proximity of the surface of such a nanostructure, the Raman signals enhanced due to the increase of the EM field because of resonant plasmons, leading to the phenomenon of SERS [14]. There are two proposed mechanisms for SERS enhancement, electromagnetic enhancement and chemical enhancement [15].
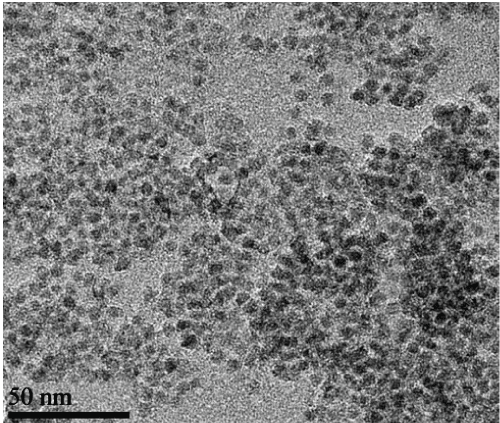 Figure 3: TEM image of silver nanoparticles used in SERS applications. View Figure 3
Figure 3: TEM image of silver nanoparticles used in SERS applications. View Figure 3
The collective excitation of the electron cloud of a conductor is called a plasmon; if the excitation is confined to the near surface region it is called a surface plasmon. EM enhancement is a consequence of the interaction of incident electric field (from incident radiation) with the electrons on the metal surface, which causes excitation of surface plasmons and, thus, enhancement of electric field at the metal surface. This mechanism was proposed by Jeanmarie and Van Duyne in 1977. Electromagnetic enhancement arises from the presence of surface plasmons on the substrate, Figure 4.
• Surface plasmons are electromagnetic waves that propagate along the surface parallel to the metal/dielectric interface.
• Surface plasmons are generated when the incident light excites the electron gas of the metal.
• When a substrate is placed in the proximity of the plasmon, it experiences an enhanced electromagnetic field and produces an enhanced scattered Raman field.
• Excitation of surface plasmon tends to form specially localized "hot areas".
• The magnitude of enhancement ~106-107 times for single colloidal silver, and ~108 for the gap between two coupled particles.
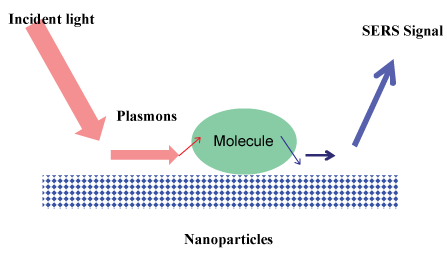 Figure 4: Interaction of laser beam with the molecules on the nanoparticles. View Figure 4
Figure 4: Interaction of laser beam with the molecules on the nanoparticles. View Figure 4
It results from an increase in molecular polarizability, due to the charge transfer between metal and sample molecule and due to specific interactions, forming charge-transfer complexes. When molecules are adsorbed to the surface, their electronic states can interact with the states in the metal and produce new transitions which cause enhancement of Raman signal. It was proposed by Albrecht and Creighton in 1977. It involves charge transfer between the chemisorbed species and the metal surface. The magnitude of chemical enhancement ~10-100 times.
SERS enhancement factor can be calculated as Analytical enhancement factor (AEF):
Where ISERS, IRS are intensities of SERS and Raman signals, respectively. CSERS, CRS are molecule concentrations for SERS and Raman, respectively.
SERS substrate enhancement factor (SSEF):
Where Nvol = CRS V-number of molecules in the scattering volume V
SERS methods are widely used for obtaining qualitative and quantitative information of different structures including pharmaceuticals. SERS line-widths are relatively narrow which allows for higher discrimination between samples with similar spectral profiles. Several pharmaceutical molecules show good Raman spectra even in diluted conditions. Commercial drugs are used in low doses and are formulated in an inert matrix or excipient to make them into a tablet form, or to modify the release rate into the patient's system. Raman mapping and imaging of samples may provide data about the distribution and relative amounts of active agent, additives, and binders present. An example is depicted in Figure 5 which shows the distribution of ketoconazole in creams samples. The spectrum of the pure pharmaceutical agent can be obtained by subtracting the matrix spectrum from that of the commercial drug. Useful spectrum may sometimes be obtained without subtraction when the pharmaceuticals are strong Raman scatters and the fillers are weak Raman scatters [15-17].
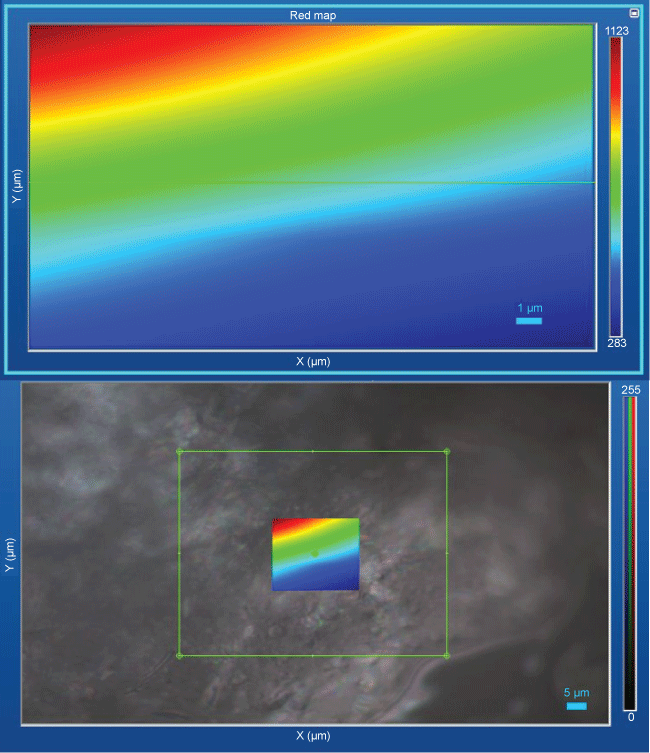 Figure 5: Mapping and image of creams samples showing the distribution ketoconazole; selected band at 1041 cm-1. View Figure 5
Figure 5: Mapping and image of creams samples showing the distribution ketoconazole; selected band at 1041 cm-1. View Figure 5
SERS allows the direct analysis of pharmaceuticals thus make its applications easy in quality control of manufacturing and formulation results in significant time and cost savings. For example, Raman spectroscopy system (a Lab Ram HR Evolution Raman spectrometer- equipped with an internal He-Ne 17 mW laser at a 633 nm excitation wavelength) was utilized for methimazole determination with silver loaded graphene dendrimer as a substrate. SERS were obtained using a small cuvette by using a volume ratio of three portions aqueous methimazole solution to one portion of nanoparticles dispersion. The data acquisition time was 20 seconds with one accumulation for collection with each SERS spectra. The SERS spectra were obtained in the range from 400-2000 cm-1 as shown in Figure 6. The method was reported with a good coefficient of determination, R2 = 0.998 with a physical detection limit of 10 pM [5].
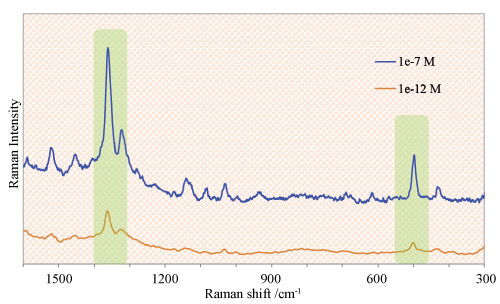 Figure 6: SERS spectra of methimazole with concentrations; 0.1 μM and 1 pM using silver nanoparticles; Laser ʎ = 633 nm, acquisition time; 30 sec, and objective; 10x. View Figure 6
Figure 6: SERS spectra of methimazole with concentrations; 0.1 μM and 1 pM using silver nanoparticles; Laser ʎ = 633 nm, acquisition time; 30 sec, and objective; 10x. View Figure 6
Eliasson and Matousek [18] demonstrated the use of spatially offset Raman spectroscopy (SORS) in the identification of counterfeit pharmaceutical tablets and capsules through different types of packaging. This method shows a higher sensitivity than that of conventional backscatter Raman spectroscopy and enables chemical information to be obtained from different depths within the sample. Davies, et al. [19] reported many polymeric materials and drug delivery systems. With high-quality spectra, drugs like promethazine, diclofenac, theophylline, and indomethacin were monitored down to the 5% (w/w) concentration level in inert polymer matrices such as sodium alginate.
By applying metal nanoparticles in SERS drug analysis, Cunningham and coworkers [20] reported a design of an optical device to identify and measure the drug contents of the fluid in an intravenous line in real time. They incorporated into the tubing a nanostructured gold substrate to observe the SERS signals of the drugs. Reported results for drugs including morphine, methadone, phenobarbital, promethazine, and mitoxantrone found to be of trace concentrations. The system has proved its capability for the fast analysis of two drugs combination solutions. The system could also be useful in urinary catheters, in hospital care, and in pharmaceutical manufacturing. Raman spectroscopy has been applied to the analysis of Chinese medicines. Feng, et al. [21] and Huang, et al. [22] reported a methodology for the detection of some traditional Chinese drugs by SERS. Intense SERS bands were observed due to the strong interaction of the drugs with the silver colloid. Thus, SERS technique has a great potential for quick, effective, accurate, and nondestructive analysis. Huang, et al. [23] reported a method for collected confocal micro-Raman spectra of chick embryo vasculature with and without the anti angiogenic drug thalidomide. The reported results indicated relative Raman intensity variations for some characteristic peaks. The reported results indicated the effectiveness of the Raman method in detecting the mechanism of vascular changes. Comparing with several methods [24-37], SERS has the advantages of being more sensitive and simple method for analysis without the need for tedious sample preparations.
Surface-enhanced Raman scattering (SERS) revolutionalised the Raman spectroscopy in the last decade. SERS is based on the marked enhancement of Raman signals of certain molecules when they are placed in the proximity of certain nanostructured metallic surfaces. Two types of mechanisms (Electromagnetic and Chemical Enhancement) are currently used to explain the SERS phenomenon. SERS has advantages of (i) High sensitivity, (ii) Specificity and Valuable tool for analyzing mixtures, (iii) Low-power lasers and low magnification optics are suitable to acquire SERS spectra in very short acquisition times (typical ~10 s), and (iv) Many applications including drug analysis, manufacturing, and quality control.
The authors would like to acknowledge the support provided by King Abdulaziz City for Science and Technology (KACST) through project No. A.T.34-8. The authors would like also to acknowledge the support by King Fahd University of Petroleum and Minerals (KFUPM).