This is a 6 year retrospective analysis of the therapeutic efficacy and safety of Combined Aliskiren (150 mg a day) and Losartan (100 mg a day) in non Diabetic Chronic Kidney Disease (CKD) patients. The objective of this Second (2nd) Phase study was to ascertain the proportion of patients who would remain in remission for the next 3 years and for those who had a relapse of proteinuria after stopping treatment in order to decide when would be an optimum time to stop therapy as well as to ascertain the effects of stopping therapy.
This is a 2nd Phase follow up study three years after the initial First (1st) Phase study. Patients in the 2nd Phase study were those who continued to have proteinuria and were treated with Losartan 100 mg a day compared to those with no proteinuria on completion of 1st Phase of the 6 year study. The 2nd Phase study seeks to document the incidence of relapse of proteinuria among the patients who had achieved a remission of proteinuria following their initial 1st Phase therapy for proteinuria.
Among the 154 patients, 67/154 (44%) continued to have proteinuria, while 87/154 (56%) had no proteinuria (remission). Of these 87 patients, 43/154 (28%) had remission with no relapse for the 3 years and the other 44/154 (29%) had relapses.
A 3 year therapy appears to be an adequate duration for Angiotensin Receptor Blocker (ARB) therapy for proteinuria and though about a third (29%) may relapse, in the majority of cases, proteinuria was less than 0.5 gm/day.
Aliskiren, Chronic kidney disease, Clinical trial follow up study
In the treatment of Chronic Kidney Disease (CKD) Angiotensin Receptor Blockers (ARBs) reduce proteinuria as well as retard the progression to end stage renal disease [1,2]. ARBs compete with the receptor for angiotensin and therefore inhibit the action of angiotensin. Aliskiren is a direct renin inhibitor which is renal protective. Renin is the rate limiting step in the Renin Angiotensin Aldosterone System (RAAS) [3]. Aliskiren allows for total blockade of the renin angiotensin system and its beneficial effect is independent of BP control [4]. One strategy which has been proven to be effective would be to employ a combination of ARB (Losartan) and Aliskiren as shown in the AVOID Trial by Parving [5]. Such a strategy would achieve the dual purpose of ARB blockade of the RAAS system.
The ALTITUDE Study based on a Combination dosage of Aliskiren and ARB was terminated because of unfavourable reports which showed that patients treated with the a Combination dosage had higher incidence of hyperkalaemia and higher incidence of strokes and myocardial infarction [6,7]. Subsequently, the Health Sciences Authority (HSA) in Singapore [8] and the European Medicines Agency [9] also issued an advisory against the use of Combination dosage of Aliskiren and ARB. When our 1st Phase I study was terminated, the results of which have been published [10], patients however continued on the study (2nd Phase ) first to see if there were any legacy effects of the 1st Phase therapy with Combination Therapy of Combined Aliskiren and Losartan and secondly to study the effects of stopping therapy on proteinuria.
Another question that remained after our initial 1st Phase study [10], which was to last only 3 years and therefore terminated inopportunely, was how long to continue any treatment if at all or should one stop therapy once proteinuria has resolved. To answer this question we designed a 2nd Phase study as a follow up for the 1st Phase study. This study hopefully would form a guide as to when we should consider stopping therapy for patients once they no longer have significant proteinuria.
In a database comprising 312 patients (Study A) with Chronic Kidney Disease attending our renal clinic, 155 patients with CKD due to Chronic Glomerulonephritis and not due to diabetic nephropathy, hypertensive nephrosclerosis, lupus nephritis or Henoch Schonlein nephritis were recruited for the study (Study B). From 2007 to July 2012, data of these 312 patients (Study A) were examined for the purpose of a retrospective study. Non biopsied CKD patients formed the bulk of our clinical practice and were more readily recruited. For purposes of standardisation of the study, we decided to recruit only non biopsied patients into the study. In this new database for the purpose of this study, the database of 155 patients (Study B) were selected, among which 51 patients were treated with combination therapy using an ARB (Losartan) and Aliskiren, 52 patients were treated with Aliskiren alone and the remaining 52 patients were treated with ARB (Losartan alone) as this was a retrospective study involving only patient medical records. Waiver of informed consent was obtained for all patients from the hospital's Institutional Review Board (IRB). Entry criteria included those patients who had been treated on the above drugs for at least 36 months within the 5 years period; other criteria included proteinuria of 1 gram or more and or Chronic Kidney Disease (CKD) Stage 3 at the start of the 36 months period. There were no significant differences in the various parameters between the 3 groups on entry into the study (Table 1). All selected patients had adequate control of BP control which was achieved with addition of atenolol, amlodipine or nifedipine. For hypercholesterolaemia, patients were treated with simvastatin or atorvastatin.
We had identified these 154 patients for a 2nd Phase study with the intention of an additional 3 year follow up with regards to documenting when proteinuria returns in some (relapse) and in the others whether proteinuria disappeared completely (Total Urinary Protein, (TUP) ≤ 0.2 gm/day) for the next 3 years without any treatment (remission). For the other patients who continue to have proteinuria they were all treated with Losartan 100 mg a day as a standard therapy and continued to be assessed every 6 months to completion of 3 years 2nd Phase follow up study (continuing proteinuria group). This practice follows the Department's guideline after the results of the ALTITUDE Trial were released and HSA issued a note of caution to the use of combination therapy with Aliskiren and an ARB in view of the reported side effects and risk of hyperkalaemia. Following this, all patients in the Department ceased usage of Aliskiren and were prescribed Losartan as a substitute.
This is a 2nd Phase follow up study three years after the initial 1st Phase study. Patients in the 2nd Phase study were those who continued to have proteinuria and were treated with Losartan 100 mg a day compared to those with no proteinuria on completion of the 1st Phase of the 6 year study (remission) who was not on any treatment. The 2nd Phase study seeks to document the incidence of relapse of proteinuria among the patients who had achieved a remission of proteinuria following their initial 1st Phase study. One patient was lost to follow up leaving 154 patients for the 2nd Phase follow up study.
Since a significant portion of the data analyses involved events like Ischemic Heart Disease (IHD) and strokes in relation to adverse events relating to drug therapy, it would be relevant to assess and compare certain factors like hypertension and hypercholesterolaemia among the three drug groups.
The incidence of hypertension in the 3 groups: Combined Aliskiren plus ARB, Aliskiren alone and ARB alone was 45% [23/51], 39% [20/52] and 57% [29/51] respectively, no significant difference. For hypercholesterolaemia the incidence was significantly lower for Aliskiren alone 42% [22/52] compared to 49% [25/51] and 67% [34/51] for the Combined Aliskiren and ARB group and for ARB alone respectively [p < 0.038].
The incidence of IHD in the Combined Aliskiren plus ARB group, Aliskiren alone and ARB alone was 20% [10/51], 12% [6/52] and 14% [7/51], showing no significant difference. Two patients had cerebrovascular accidents (lacunar infarction) among the ARB alone group but none in the other 2 groups. The difference was not significant.
All 155 patients on the database had the following investigations documented at six monthly intervals: serum creatinine, eGFR and Total Urinary Protein (TUP). Serum creatinine was quantitated with alkaline picrate and TUP was quantitated by biuret agent. Estimated Glomerular Filtration Rate (eGFR) was estimated using the Cockcroft Gault formula for eGFR. Decrease in eGFR was expressed as ml of eGFR loss per year over the 6 year duration from time of entry to exit of the trial. Improvement in eGFR was taken as the positive difference between the entry eGFR and the exit eGFR over the study period. End stage renal failure was equated with decline of eGFR to CKD stage 5 with eGFR less than 15 ml/min/year. The primary end points were stage 5 CKD or end stage renal failure. The secondary end points were reduction of proteinuria by 50% and change in eGFR.
For the 1st Phase study, the 155 patients with CKD were on various combinations of Aliskiren with Losartan, Aliskiren alone or Losartan alone for a period of 3 years. As the patients were not randomised on these various drugs, our conclusions would need to be conservative and this would be a limitation of the study. In the 2nd Phase, Losartan 100 mg daily was the treatment for those patients who had persistent proteinuria following the end of the 1st Phase study.
The 2nd Phase follow up study was for three years after the initial 1st Phase study. Patients in the 2nd Phase study were those who continued to have proteinuria and were treated with Losartan 100 mg a day compared to those with no proteinuria on completion of 1st Phase study.
Sample size calculation was based on the proportion of patients achieving 30% decrease in TUP with treatment of normal dose Aliskiren or normal dose Losartan. A second sample size calculation was done to compare the rate of 30% TUP decrease between a combination dose of ARB plus Aliskiren and Aliskiren alone. Assuming that the rate of TUP decrease to be 30% in the Normal dose ARB and Normal dose Aliskiren and 60% in the combination dose of ARB plus Aliskiren, the number of patients required in each group was 49 for a 2-sided test with alpha = 0.05 and power of 80%. We expected the effects of combination dose of ARB plus Aliskiren to be about the same as that of High dose ARB. Sample size for Phase II is 154 patients as 1 patient was lost to follow up due to emigration.
SPSS 10.1 for Windows was used for all analysis. Results were expressed as mean ± SD or median (range) or count (%). For univariate analysis, Pearson's chi-square test was used for comparing categorical data and ANOVA for comparing numeric data between the 3 treatment arms. ANOVA was followed by multiple comparison with Student-Newman-Keuls (SNK) range test whenever statistical significance was found between the 3 treatment arms as well as the three arms of patients with remission, relapse and continuing proteinuria.
Next, a doubly Multivariate ANOVA (MANOVA) with repeated measures was used to test the effect of drug treatment on both eGFR and Total Urine Proteinuria (TUP). The dependent variables were eGFR and TUP measured at 7 time points, namely baseline and thereafter every year of the 6 years of the study. The between-subject factor was treatment group with 3 levels corresponding to Combination dose of ARB and Aliskiren, Aliskiren alone and ARB alone. This was repeated for the other 3 patient arms of remission, relapse and continuing proteinuria. Adjustment was made for the covariates of average systolic BP and average diastolic BP. Average blood pressures were calculated by taking the mean of all blood pressures while on medication (mean of blood pressures from year 1 to year 6). Within MANOVA, the effect of combination dose of Aliskiren and ARB on the outcomes of eGFR and TUP was compared with each of the other drug dosage groups by simple contrast comparison testing. Similarly, repeated contrast testing was done to obtain and compare the loss in eGFR in each year between the various drug groups. The same MANOVA was repeated for the three patient arms of remission, relapse and continuing proteinuria.
Plots of mean values of eGFR and TUP adjusted for covariates of systolic BP and diastolic BP were presented; so were the contrast estimates, their corresponding 95% confidence intervals and p-values for the comparison of eGFR and TUP between the levels of interest of the treatment group as well as the patients with remission, relapse and continuing proteinuria.
Table 1 compares the demographic and clinical profile of patients treated with combined dose of Aliskiren and Losartan, Aliskiren alone and Losartan alone from year 1 to 6. The eGFR and TUP was significantly lower in all 3 arms before and after the trial and the decrease was not significantly different among the 3 arms for eGFR.
Table 1: Comparing demographic and clinical profile of patients treated with combined dose aliskiren and losartan, aliskiren alone and losartan alone (Year 1 to 6). View Table 1
Figure 1 compares the eGFR and decrease in eGFR between Combination dose of Aliskiren and ARB, Aliskiren alone, and Losartan alone, before and after the trial. The decrease in eGFR per year was not significantly different among the 3 arms. There were no patients with ESRD at the end of the study in all the 3 groups.
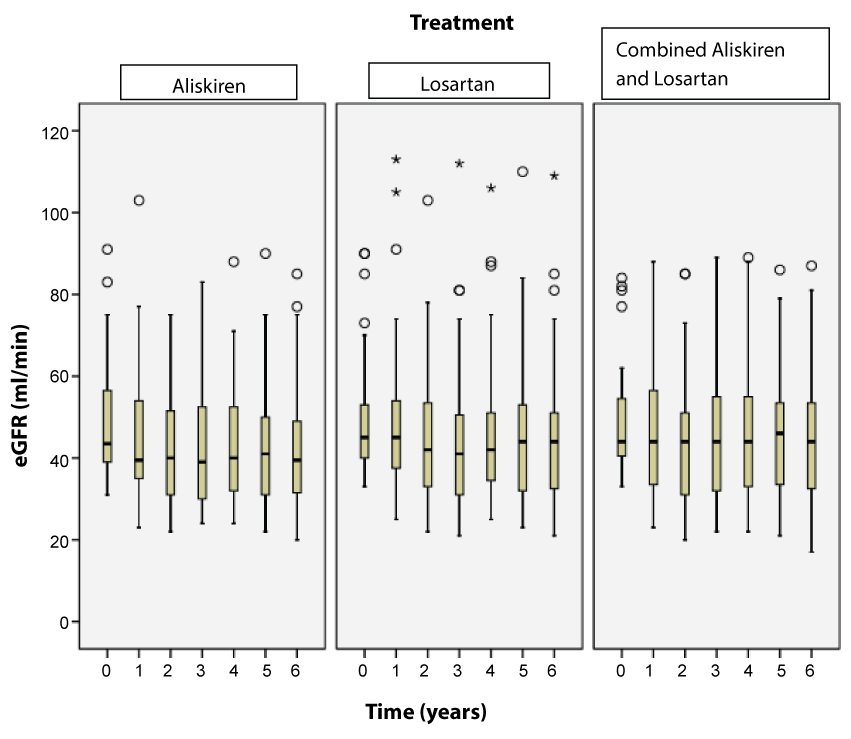 Figure 1: Distribution of eGFR (ml/min) over the years by treatment arm.
Comparison of eGFR over the whole 6 year period between the 3 treatment arms by MANOVA: p = 0.478. The distribution of eGFR is shown in boxplots. The lower boundary of box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of eGFR respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 1
Figure 1: Distribution of eGFR (ml/min) over the years by treatment arm.
Comparison of eGFR over the whole 6 year period between the 3 treatment arms by MANOVA: p = 0.478. The distribution of eGFR is shown in boxplots. The lower boundary of box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of eGFR respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 1
Figure 2 shows the distribution of Total Urinary Protein (TUP) over the years by treatment arm. TUP was lower at the end of the study in all 3 arms. The changes in TUP showed a reduction between the baseline and each year with the Losartan group showing the greatest reduction in proteinuria (p = 0.016) (Table 1 and Figure 2).
 Figure 2: Distribution of total urinary protein (g/day) over the years by treatment arm.
Significant difference in TUP over the whole 6 year period between the 3 treatment arms by MANOVA (p = 0.011 for treatment). At each time point except baseline, TUP was lower for Aliskirin compared to Combined Aliskirin and Losartan (p < 0.05 at each time point). The distribution of TUP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of TUP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values. View Figure 2
Figure 2: Distribution of total urinary protein (g/day) over the years by treatment arm.
Significant difference in TUP over the whole 6 year period between the 3 treatment arms by MANOVA (p = 0.011 for treatment). At each time point except baseline, TUP was lower for Aliskirin compared to Combined Aliskirin and Losartan (p < 0.05 at each time point). The distribution of TUP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of TUP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values. View Figure 2
At each time point except for baseline, TUP was lower for Combined Aliskiren and Losartan compared to Aliskiren alone (p < 0.05), and by MANOVA the p value was < 0.011. The TUP for Combined Aliskiren and Losartan compared to Losartan alone was not significantly different at each time point.
A Chi Square analysis of the 3 drug arms compared with the proportion (%) of patients in each of the groups, show that those patients treated with Combined Aliskiren and Losartan had more remission (37%), most relapses (45%) and least number of patients with continuing proteinuria (22%) (p < 0.043) as shown in Table 1.
Table 2: Comparing demographic and clinical profile of patients with remission, relapse and continuing proteinuria (Year 1 to 6). View Table 2
The BP levels, Systolic and Diastolic over the 6 years for the 3 drug groups are displayed in Figure 3 and Figure 4 respectively. There were no significant differences between the 3 drug groups throughout the 6 years.
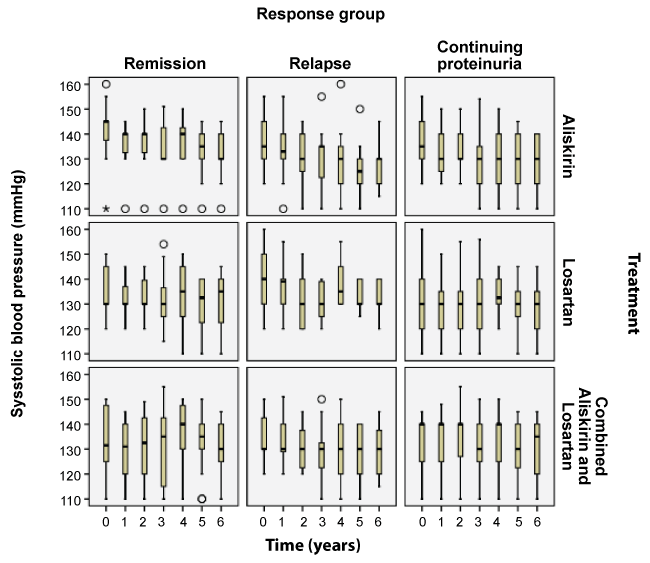 Figure 3: Distribution of systolic blood pressure (mmHg) over the years by combination of treatment arm and response group at the end of trial.
The distribution of SBP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of SBP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 3
Figure 3: Distribution of systolic blood pressure (mmHg) over the years by combination of treatment arm and response group at the end of trial.
The distribution of SBP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of SBP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 3
 Figure 4: Distribution of diastolic blood pressure (mmHg) over the years by combination of treatment arm and response group at the end of trial.
The distribution of DBP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of DBP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 4
Figure 4: Distribution of diastolic blood pressure (mmHg) over the years by combination of treatment arm and response group at the end of trial.
The distribution of DBP is shown in boxplots. The lower boundary of the box, black horizontal line inside box and the upper boundary of the box represent the 25th percentile, median and 75th percentile of DBP respectively. A circle (⚪) denotes an outlier which by definition is any case 1.5 × IQR away from either end of the box, an asterisk (*) an extreme value which is a case 3 × IQR away from either end of the box. IQR: Interquartile Range. The whiskers that are lines extending beyond the box correspond to the smallest and largest values that are neither outliers nor extreme values.
View Figure 4
Table 2 compares the demographic and clinical profile of patients with Remission (X), Relapse (Y) and Continuing Proteinuria (Z). The eGFR in all 3 groups continue to decline but there were no significant differences between the 3 groups. There was a significant difference in the reduction of proteinuria in all 3 groups (p < 0.001). The TUP was 0.2 ± 0.2 gm/day for those in remission, 0.5 ± 0.4 gm a day for those with relapses and 0.5 ± 0.5 gm a day for those with continuing proteinuria. The distribution of the degree of proteinuria is shown in Figure 5 for patients in remission (X), Figure 6 for those with relapses (Y) and Figure 7 for those with continuing proteinuria (Z). There were 43 patients with remission (X) lasting for the 3 years of the Phase II study, 44 patients with relapses (Y) but most were less than 1 gm a day of proteinuria, only 2 patients had TUP 1.2 gm and 1 gm a day during the Phase II study period of 3 years. Among those with continuing proteinuria (67 patients) (Z), the maximum was 3 gm a day but mean proteinuria 0.5 ± 0.5 gm/day in patients maintained on Losartan 100 mg a day.
 Figure 5: Distribution of proteinuria for patients in remission. There were 43 patients with remission (X) lasting for the 3 years of the Phase II study. TUP 4-7 refers to Total Urinary Proteinuria for year 4 to 7. View Figure 5
Figure 5: Distribution of proteinuria for patients in remission. There were 43 patients with remission (X) lasting for the 3 years of the Phase II study. TUP 4-7 refers to Total Urinary Proteinuria for year 4 to 7. View Figure 5
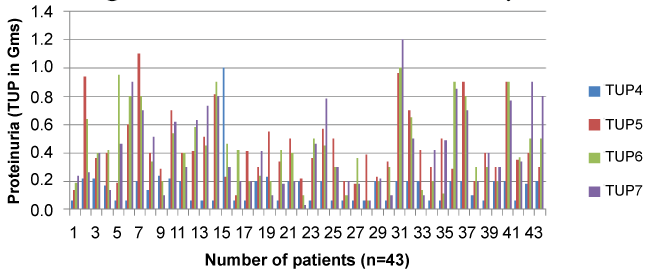 Figure 6: Distribution of Proteinuria for patients with relapses. 44 patients with relapses (Y) but most were less than 1 gm a day of proteinuria.
View Figure 6
Figure 6: Distribution of Proteinuria for patients with relapses. 44 patients with relapses (Y) but most were less than 1 gm a day of proteinuria.
View Figure 6
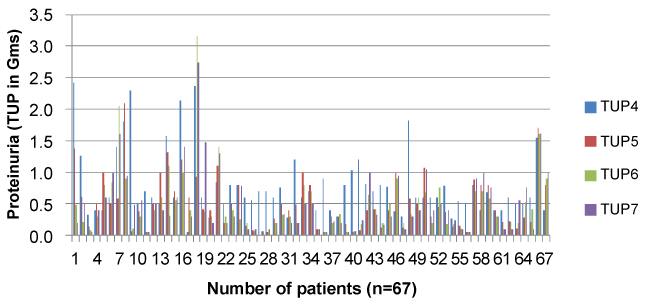 Figure 7: Distribution of proteinuria for patients with continuing proteinuria. Among those with continuing proteinuria (67 patients) (Z), the maximum was 3 gm a day but mean proteinuria was 0.5 gm a day. View Figure 7
Figure 7: Distribution of proteinuria for patients with continuing proteinuria. Among those with continuing proteinuria (67 patients) (Z), the maximum was 3 gm a day but mean proteinuria was 0.5 gm a day. View Figure 7
In the 1st Phase study in 155 patients with CKD over 3 years we showed that the use of Combination therapy of Aliskiren with ARB was not more efficacious as an antiproteinuric drug when compared to Aliskiren or ARB alone.
In the present 2nd Phase study, the same patients were followed up for another 3 years to observe the effects of stopping therapy on proteinuria.
TUP was lower at the end of the 6 year study in all 3 arms. The changes in TUP showed a reduction between the baseline and each year with the Losartan group showing the greatest reduction in proteinuria. At each time point except for baseline, TUP was lower for the patients who had been in the Combined Aliskiren and Losartan arm for the prior 3 years compared to those who had been in the Aliskiren arm. It would appear from the 2nd Phase study that there seemed to be a more efficacious effect of the Combination therapy of Aliskiren and Losartan compared to Aliskiren alone. The addition of Losartan to Aliskiren seemed to have caused a further reduction in proteinuria. The TUP for Combined Aliskiren and Losartan compared to Losartan alone, however, was not significantly different at each time point.
Parving, et al. in 2008, published the results of a double blind randomised controlled trial of Aliskiren combined with Losartan in 599 patients with Type 2 Diabetes with nephropathy [AVOID Study] [5] over a 6 months period. The results of the study showed that the decline in eGFR was the same in the treatment and placebo group but the decline in the treatment group tended to be less than in the placebo group at 6 months. The reduction of albuminuria by 50% occurred twice as often in the treatment group compared to the placebo group. The authors concluded that Aliskiren appeared to have a renoprotective effect independent of its BP lowering effect in patients with Type 2 diabetes who were receiving maximal renoprotective treatment and optimal antihypertensive therapy. Persson, et al. [11] in a post hoc analysis of Parving's AVOID trial [7] concluded that Aliskiren added to Losartan reduced albuminuria and renal dysfunction in diabetics.
In an open labelled pilot study by Tang, et al. [12] in 25 consecutive patients where Aliskiren [300 mg/day] was prescribed despite being on maximum ARB therapy with Losartan [100 mg/day] for 3 months in patients with IgA nephropathy [stage 3 CKD with proteinuria > 1 gm/day] over a 12 month period. There was a 22% reduction in proteinuria at 6 months and a 26% reduction at 12 months. The authors concluded that Aliskiren conferred an antiproteinuric effect in patients with IgA nephropathy with significant residual proteinuria, despite receiving the recommended renoprotective treatment.
The next issue concerns the effects of stopping treatment of proteinuria. Among the 154 patients in the 2nd Phase study, 43 out of 154 (27%) patients could stop treatment without recurrence of proteinuria for 3 years. Another 44 out of 154 (29%) patients had no proteinuria but had relapsed during the 3 years follow up 2nd Phase study, but proteinuria was mild and only 2 patients had proteinuria of 1 gm and 1.2 gm each.
A 3 year therapy appears to be an adequate duration for therapy for proteinuria and though about a third of patients (29%) may relapse, in the majority of cases, proteinuria was less than 0.5 gm/day with only 2 patients with TUP about 1 gm. This study could support the concept that patients should not continue to be on long term therapy with ARB after being free of proteinuria for 3 years.
Most guidelines for therapy of proteinuria [13,14] advise the prescription of ACEI/ARB for patients with CKD with proteinuria in excess of a gram a day. In our study, patients had moderate proteinuria TUP of 1.4 gm/day for the Aliskiren group on initiation into the trial and would fulfil the criterion for therapy with ACEI/ARB therapy.
Our previous study [12] in 155 patients with CKD over 3 years showed that the use of Combination therapy of Aliskiren with ARB was not more efficacious as an antiproteinuric drug when compared to Aliskiren or ARB alone at the end of 3 years (1st Phase study). This 2nd Phase study showed that a 3 year therapy appears to be an adequate duration for therapy for proteinuria and though about one third of patients (29%) may relapse, in the majority of cases, proteinuria was less than 0.5 gm a day. This study could support the concept that patients should not continue to be on long term therapy with ARB after being free of proteinuria beyond the initial 3 years.
This is an Academic Trial supported by Singhealth Cluster with IRB approval, CIRB Ref: 2016/2586. There was no grant support or other financial assistance involved in the study. All the authors declared no competing interests. We would like to acknowledge M/s Irene Ow for administrative support.