Objectives: This narrative literature review provides an overview of the different strategies that have been successfully used to reduce the dose of intravenous contrast media (ICM) while maintaining image quality of pulmonary arteries in computed tomography pulmonary angiography (CTPA). These strategies include optimizing the ICM dose, utilizing modern CT scanners capabilities, customizing patient-specific protocols, and using advanced image reconstruction techniques.
Materials and methods: Thirteen relevant studies published up to February 2024 were identified across PubMed and Scopus databases using a comprehensive search strategy that employed the search terms "CTPA," "contrast," "reduction," and "minimization." An additional manual search on the Research Gate platform identified eight more studies, which were included in the qualitative synthesis. The inclusion criteria focused on studies that compared image quality between CTPA protocols with reduced ICM dose and standard ICM dose CTPA, or CTPA protocols with different ICM doses.
Conclusions: The review revealed several strategies, including the optimization of ICM dose, leveraging the capabilities of modern computed tomography scanners, patient-specific protocol customization and advanced image reconstruction techniques, which have been successfully implemented to reduce ICM dose while maintaining image quality of pulmonary arteries in CTPA. In addition, scanning with low kVp has allowed reduction in both the required ICM dose and the radiation dose to the patient. Conclusively, reducing the dose of administered ICM in CTPA is feasible, with several techniques and protocols demonstrating efficacy in clinical settings.
Computed tomography pulmonary angiography, Pulmonary embolism, Iodinated contrast media
In recent years, advancements in medical imaging technology have revolutionized the field of diagnostics. Computed Tomography Pulmonary Angiography (CTPA) is a widely accepted diagnostic standard [1] for identifying and evaluating blood clots within the pulmonary arteries (PA) and has a higher sensitivity and specificity than the D-dimer test alone [2]. However, the use of iodinated contrast media (ICM) in contrast-enhanced imaging procedures requires caution to ensure patient safety and diagnostic accuracy. As the global healthcare community increasingly embraces a commitment to patient safety, there is a growing need to explore and implement practices, that promote patient safety within diagnostic imaging [3,4]. By optimizing scan parameters, refining imaging techniques and leveraging the technological advancements of modern CT scanners, radiological departments can significantly reduce the dose of ICM administered without compromising image quality in CTPA studies.
While ICM administration is necessary to obtain contrast-enhanced images of the PA, its use is not without potential risks and complications for the patient. Conditions such as food, drug and contrast-induced allergies, hyperthyroidism, diabetes, chronic kidney disease and multiple myeloma should be documented and considered before the ICM administration, to avoid allergic reactions [5], contrast-induced nephropathy (CIN) [6], anaphylaxis [7], thyrotoxicosis [8] and cardiac arrhythmia [9], which can be even fatal for the patient. Additionally, ICM can impose an increased burden or even cause post-contrast acute kidney injury in patients with compromised renal function [10]. The literature demonstrates an interest in optimizing the dose of intravenous ICM used in CTPA, aiming to achieve a balance between image quality and patient safety. Moreover, by analyzing the current research landscape, this review aims to highlight the efficacy of low ICM dose imaging techniques in CTPA with respect to image quality and summarize their outcomes.
Pulmonary embolism (PE) occurs when a blood clot in a deep vein in lower extremities or pelvis is detached and travels through the blood circulation to the lungs, blocking the blood supply from the heart to the lungs. It is a common and serious medical condition with potentially fatal consequences for the patient. PE can present with a variety of symptoms, including shortness of breath, chest pain, rapid heart rate and cough [11]. However, the clinical presentation can be nonspecific, making the diagnosis challenging [12]. CTPA has become the standard imaging modality for diagnosing PE due to its high sensitivity and specificity [2]. It offers several advantages, including its non-invasive nature, rapid imaging acquisition, and high diagnostic accuracy. CTPA allows for the prompt identification and localization of pulmonary emboli from radiologists, guiding clinicians in making timely and informed decision-making [13]. Clinical guidelines recommend CTPA as the first-line imaging test for suspected PE, particularly in cases where the clinical probability is moderate or high, making the use of CTPA integral to the diagnostic algorithm for PE [14]. During CTPA, an ICM bolus is injected into the patient's peripheral vein or through a central venous access using a power injector. The injected ICM enhances the opacification of the main and peripheral PA, while the CT scanner acquires transversal images of the chest, which can be submitted in multiplanar and three-dimensional reconstructions for the detailed imaging of the PA [15]. Appropriate injection rate and technique, scan delay and scan timing are crucial parameters, which allow the radiology team to synchronize the scan with the peak of the contrast-enhancement in PA and achieve optimal CTPA studies [16].
Beyond diagnosis, CTPA plays a crucial role in risk stratification and treatment planning. It facilitates determine the extent of PE, guiding decisions on anticoagulant therapy and, in severe cases, interventions such as thrombolysis or embolectomy [14]. CTPA has revolutionized the diagnosis of PE, providing radiologists and clinicians with a powerful and efficient tool for accurate visualization of the PA. Its widespread adoption is reflective of its diagnostic efficacy and its pivotal role in improving patient outcomes through timely and appropriate management of PE, with the contribution of radiographers to CTPA protocols optimization and manipulation of CT parameters to be considered significant, in order to obtain high quality images [17].
The age and clinical condition of the patient, as well as coexisting medical conditions, such as cardiovascular diseases, diabetes, and chronic kidney disease, can affect vascular anatomy, cardiac output, breathing, mobility and cooperation, making CTPA a challenging procedure in some cases. Some patients may have mobility issues, cognitive impairments, or encounter difficulties that need to be addressed, to ensure a successful and comfortable imaging experience [18]. In addition, the radiology team must be aware of the patient’s hemodynamics, to ensure sufficient imaging of the PA. Changes in heart rate and blood circulation may vary with age, impacting the administration of ICM and timing considerations of image acquisition and contrast enhancement in CTPA for different age groups [19].
The body mass and weight of the patient are key factors in CTPA, influencing ICM dose, image quality, radiation dose and diagnostic accuracy. Larger individuals may require higher ICM doses and radiation exposure to achieve optimal PA enhancement [20]. Additionally, the injection rate may be adjusted based on the patient's body mass to ensure adequate contrast delivery [21]. Patients with increased body mass may require an increase in tube current and voltage, to optimize image quality and maintain diagnostic accuracy. Obesity can pose an increased burden on the diagnostic accuracy of CTPA, affecting the visualization of the PA [22]. The radiology team may need to employ specific imaging techniques or reconstruction algorithms to overcome these challenges in patients with an increased body mass.
Adopting a low ICM dose protocol contributes to mitigate the risk of adverse reactions and CIN [23]. These protocols involve lower dose or concentration of contrast agents compared to routine CTPA protocols while maintaining diagnostic image quality. Tailoring ICM doses based on patient-specific factors, including weight and clinical history, is part of individualized imaging protocols. CTPA protocols with individualized injection parameters of ICM may be feasible, providing sufficient image quality with a substantial radiation dose reduction [24]. In addition, weight-based ICM dosing may ensure that each patient receives adequate ICM for optimal imaging [20].
Several technological advancements have revolutionized Computed Tomography (CT), offering radiologists and radiographers new capabilities to perform faster CT scans and minimize radiation and ICM exposure to the patient. Multi-detector (MDCT) and dual-source CT (DSCT) scanners have made significant contributions to the reduction of ICM volume in CTPA. Allowing faster scanning, improved spatial and temporal resolution, dual-energy (DE) scanning options, advanced reconstruction algorithms and monochromatic imaging options, these CT scanners have assisted radiologists, radiographers and researchers in reducing both radiation exposure and ICM volume, without any compromise in image quality compared to routine CTPA protocols [25-28]. Studies have recorded that a low tube voltage (kVp) CTPA protocol on a MDCT scanner may allow simultaneous reduction of radiation exposure and ICM volume while maintaining image quality [29]. On the other hand, studies investigating the efficacy of CTPA protocols employing either low iodine concentration agents or low ICM volume on DSCT scanners have concluded that these scanners may produce high-quality CTPA images alongside a significant reduction in iodine load for the patient [30-32]. Several studies have also investigated high-pitch CTPA protocols. High-pitch scanning offers several benefits during scanning, including reduced scan times and decreased radiation dose compared to standard scanning methods. These capabilities are particularly valuable when fast image acquisition and motion artifact reduction are essential, such as in CTPA [33-36]. Novel technological advancements in CT hardware, such as photon-counting detectors are promising, allowing for significant reduction of ICM and radiation dose in the diagnosis of PE, while maintaining good to excellent image quality [34,37].
Advanced reconstruction algorithms play a pivotal role in maintaining image quality while reducing ICM volume. Iterative reconstruction techniques, in particular, enable image noise reduction and enhance image spatial resolution, compensating for lower exposure parameters and contrast densities [28]. Dual-energy (DE) monochromatic image reconstruction can improve the overall signal-to-noise ratio (SNR), allowing high diagnostic quality even with lower ICM volume or iodine concentration [30]. These advances can compensate for low iodine concentration and reduced volume of ICM, ensuring that image quality in CTPA remains uncompromised.
This review was conducted according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines [38]. PubMed and Scopus databases were searched in February 2024 without any time restriction for comparative studies and clinical trials that measured image quality of reduced ICM dose CTPA protocols, using the terms: “CTPA” combined with the term “contrast” and either with the term “reduction” or “minimization”. Any duplicates from the two searches were merged. On an additional manual search on the Research Gate platform, eight more studies were identified and included in the qualitative synthesis. As part of the screening process; any records that were not relevant to the subject of this review were removed.
Full-text articles were considered for eligibility if they met the following inclusion criteria (Table 1).
Table 1: Inclusion and exclusion criteria for study selection. View Table 1
Full-text articles not meeting those criteria were excluded. The procedure of study selection is depicted in the flow diagram in Figure 1 and the data of the twenty-one studies that fulfilled the study criteria are summarized in Table 2.
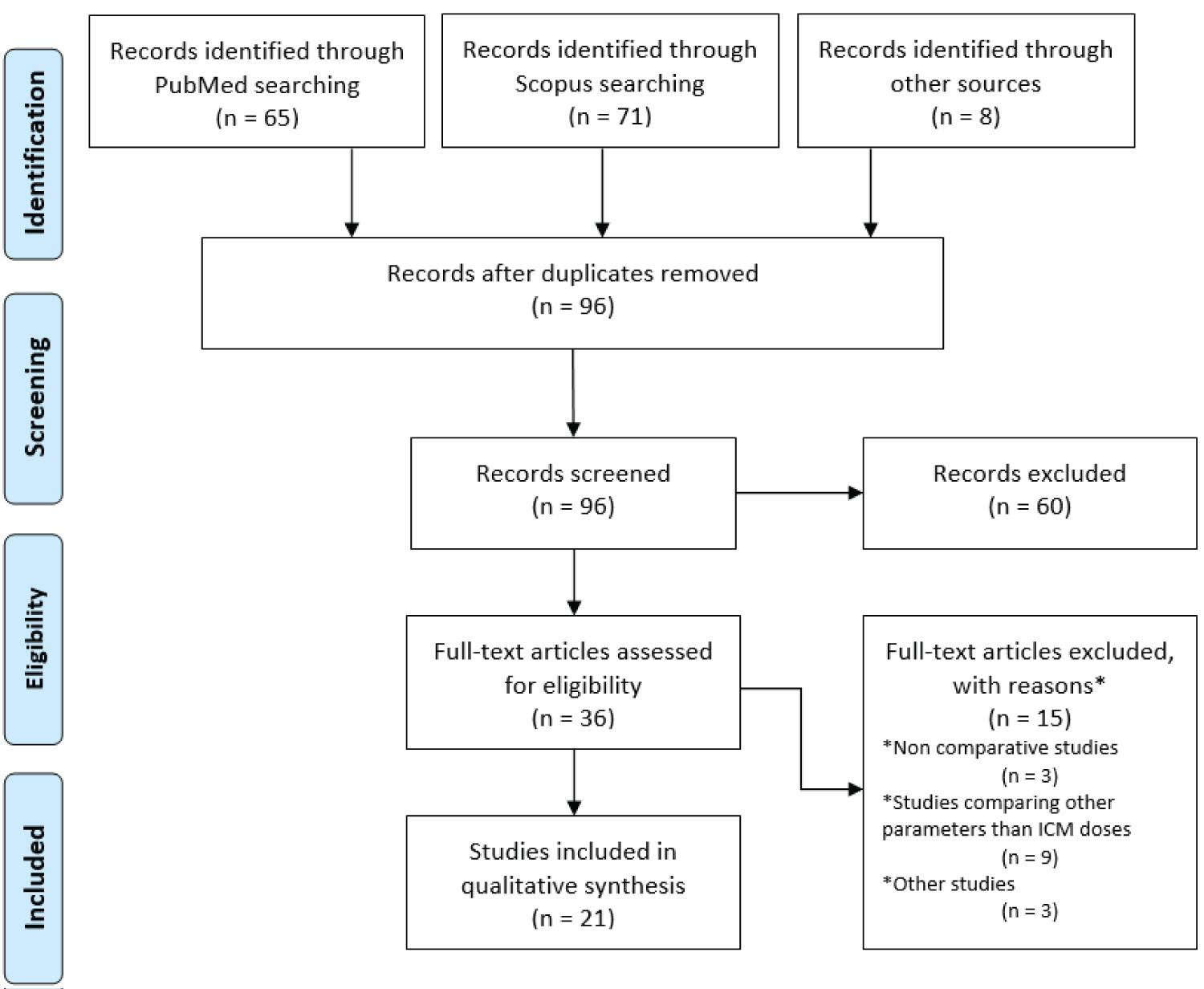 Figure 1: PRISMA flow diagram of study identification and selection.
View Figure 1
Figure 1: PRISMA flow diagram of study identification and selection.
View Figure 1
Table 2: Summary of the characteristics of studies included in the review (published 2010-2023). View Table 2
This review includes 21 studies, both prospective and retrospective, that were published between 2010 and 2023. These studies involved 3000 adult patients suspected of having PE who underwent CTPA. Most studies used both qualitative and quantitative image evaluation to compare the image quality of different CTPA protocols with different ICM doses. However, in three studies, only qualitative or quantitative image evaluation was used. Most studies evaluated both subjective and objective image quality. The subjective evaluation relies on the interpretation of images by radiologists or trained observers, focusing on the opacification of PA, image noise levels and artifacts, which directly influence diagnostic confidence in CTPA. The observers used rating scales to rate the vasculature opacification across the included studies. The rating scales varied from 3 to 5-point, where the first number denotes excellent image quality and the last number denotes non-diagnostic image quality. On the other hand, objective evaluation employs quantitative metrics to measure image characteristics such as CNR and SNR, providing a standardized means to compare imaging protocols. While objective measures offer reproducibility and can guide protocol optimization, they may not fully capture the diagnostic utility perceived by human observers. Thus, a comprehensive assessment of CT image quality necessitates a balanced integration of both subjective and objective evaluations, ensuring that imaging techniques not only meet technical standards but also effectively support clinical decision-making. All studies presented in Table 2 demonstrate feasible CTPA protocols with reduced ICM doses.
In total, fifteen full text articles were excluded from the qualitative synthesis. Three studies assessed the efficacy of a CTPA protocol utilizing a low ICM dose, however, none of them conducted a comparative analysis with a standard protocol [28,35,39]. Nine studies were investigating other parameters or aspects of CTPA protocols or contrast agents, such as injection rate and iodine concentration [31,40-47]. One study was a phantom study [48]. One study examined the diagnostic accuracy of a CTPA protocol with low radiation and ICM dose compared to a standard protocol for different body weights without assessing image quality [26]. One study compared a weight-adjusted contrast administration protocol to a standard CTPA protocol, using various ICM doses instead of using only a low dose of ICM [20].
Achieving acceptable diagnostic image quality in CTPA with lower ICM dose involves a wide range of protocols and techniques, as well as utilizing the capabilities of the modern CT scanners and injectors. Several studies have demonstrated the effectiveness of simultaneous high-pitch technique with reduced ICM dose in maintaining image quality. High-pitch CTPA with low dose of ICM and reduced radiation dose may render comparable subjective image quality to standard CTPA with sufficient PA contrast opacification and CNR above diagnostic thresholds in most cases, despite the reduced objective image quality of this technique compared to standard CTPA [33]. At significantly low kVp the use of novel image reconstruction methods such as Sinogram Affirmed Iterative Reconstruction (SAFIRE) may provide comparable image quality and substantial radiation dose reduction compared to a standard CTPA protocol with filtered-back projection reconstruction [58,63].
As evidenced by researchers, novel Photon-Counting Detector (PCD) technology in CT scanners has facilitated the reduction of ICM dose in CTPA even below 60 ml, providing acceptable image quality [34]. PCD-CTPA offers substantial advantages in terms of rapid image acquisition, radiation and ICM dose reduction and advanced imaging methods. The virtual mono energetic imaging on PCD-CT scanner offers the potential of ICM dose minimization and allows good to excellent image quality compared to a standard DE-CTPA protocol [37].
DE-CTPA presents another approach to reduce ICM dose and iodine load. Researchers, who compared DE-CTPA with standard CTPA, demonstrated that reduced iodine load [53] or ICM dose [49] does not significantly affect the opacification of PA, offering a potential path toward minimizing ICM use. Although aDE-CTPA protocol may result in high SNR and CNR, a slight reduction in image quality and increased image noise may be noted in some cases [30].
Many researchers have denoted the significance of individualized CTPA protocols, tailored to patient’s characteristics such as body weight [20]. A significant reduction in ICM dose was achieved through weight-adjusted contrast administration, as evidenced by Hendriks, et al. who emphasized the importance of tailoring ICM dose to patient weight [56]. They concluded that an individualized CTPA protocol can provide diagnostic image quality with a substantial reduction of ICM volume, especially for lower weight patients, compared to a CTPA protocol with a fixed ICM dose for these patients.
Exploring the feasibility of simultaneous low kVp and ICM dose, researchers have demonstrated the efficacy of such CTPA protocols with significantly reduced contrast dose and radiation exposure, maintaining sufficient image quality to exclude or diagnose PE. Suntharalingam, et al. have concluded that their submillisievert standard-pitch CTPA protocol with 25 ml ICM dose may obtain sufficient image quality while reducing radiation dose by approximately 71% compared to a standard protocol [54]. According to Szucs-Farkas, et al. reduced radiation and ICM dose can provide high vessel attenuation, while maintaining diagnostic image quality and diagnostic confidence [25]. Sodickson and Weiss have also demonstrated the efficacy of their CTPA protocol with low kVp and an appropriately designed ICM injection, to obtain diagnostic images, while reducing radiation exposure and ICM dose by 33% [29].
The concentration of ICM plays a key role in optimizing image quality in reduced ICM dose CTPA protocols. Higher concentrations of ICM allow for reduced volumes to be used while still achieving the necessary PA opacification for diagnostic imaging. This is particularly important in protocols aiming to minimize the dose of ICM to reduce the risk of nephrotoxicity and allergic reactions in vulnerable patients. Goble and Abdulkarim have demonstrated that a reduced volume of high-concentration ICM combined with multiphasic injection technique; allow contrast dose reduction without compromising CTPA accuracy [60]. Furthermore, the combination of low kVp and high-concentration ICM enables further reduction in contrast dose while maintaining or enhancing image quality, underscoring the importance of ICM concentration in achieving optimal diagnostic outcomes with minimal patient risk.
Reducing contrast agents is crucial for ensuring patient safety. Iodinated contrast agents can result in both mild and severe allergic reactions. Mild reactions include symptoms like rash, nausea, and itching, while severe reactions can lead to pulmonary edema, cardiac arrhythmia or arrest. To minimize the likelihood of triggering allergic responses, it is important to reduce the use of injected contrast agents. This is particularly important for patients with a history of allergies to contrast agents, food or drugs [5].
One of the most significant concerns associated with ICM is the development of contrast-induced nephropathy (CIN), particularly in individuals with chronic renal disease [64]. CIN is a serious complication of angiographic procedures following the administration of ICM and is characterized by a sudden impairment of kidneys function. Minimizing the dose or iodine load of the injected contrast agent, especially in patients with renal impairment, can reduce the risk of CIN [65].
Excessive iodine exposure can also affect thyroid function. Minimizing the use of ICM is particularly important for individuals with thyroid disorders like hyperthyroidism, or those at risk of developing thyroid dysfunction. Close monitoring and consideration of alternative imaging approaches may be warranted in such cases, to avoid thyroid dysfunction or thyrotoxicosis [8].
For patients who undergo multiple imaging procedures over time, the cumulative dose of ICM becomes a concern. Minimizing ICM exposure helps mitigate the potential of accumulating high doses of iodine, causing organ dysfunction, triggering allergic reactions and increasing the absorbed organ radiation dose [66], which could lead to contrast-induced adverse effects and risk of cancer.
Extravasation, the unintended leakage of an intravenously injected contrast agent into surrounding tissues, can cause mild skin reaction like inflammation or erythema, and more severe complications such as skin ulceration, tissue necrosis, and compartment syndrome [67]. Managing each patient according to her or his needs, establishing optimal venous access, minimizing the volume, injection rate and concentration of contrast agents, as well as employing proper injection techniques where needed, can reduce the potential of extravasation during CTPA [68,69].
Pregnant women are particularly vulnerable to the potential risks of ionizing radiation exposure. If a pregnant patient must undergo a CTPA, appropriate scan protocol selection and optimization of scan length may be needed, to minimize exposure of the patient and the developing fetus, whilst maintaining diagnostic quality [70]. Despite the use of ICM is considered safe during pregnancy [71], radiology professionals should carefully weigh the benefits of contrast-enhanced imaging against the potential risk of fetal hypothyroidism and submit pregnant patients in CTPA only when the clinical situation requires doing so, keeping the volume of ICM administered as low as possible [72].
Some patients may experience stress, anxiety or discomfort before or during CTPA. Sudden pain at the region of intravenous access, warmth or cold sensation during the administration of ICM may extend the patient’s discomfort [73]. Vulnerable populations, such as pediatric patients, the elderly, and those with multiple comorbidities, may be at a higher risk of contrast-induced adverse effects. Therefore, warming and tailoring ICM volume to the specific needs of these populations may reduce the risk of extravasation or allergic reactions, enhancing overall safety and contributing to a more positive patient experience, especially in cases where the diagnostic benefits of contrast-enhanced phase may be marginal [74]. Providing the patient with detailed information about the CTPA procedure and its potential risks, coupled with maintaining open communication throughout the ICM injection, is essential. This ensures that the patient feels safe and at ease, while simultaneously allowing the healthcare professional to monitor the patient effectively during the procedure [75]. In summary, healthcare professionals must carefully conduct a thorough screening of patients suspected of PE to assess their medical history, including any history of allergies, renal function, and pregnancy status, to determine the necessity of contrast-enhanced imaging, weighting the potential risks against the diagnostic benefits.
The investigation of reducing ICM dose in CTPA reflects a multifaceted approach aimed at optimizing patient care, diagnostic accuracy and patient safety. Numerous studies have delved into the clinical efficacy of CTPA with reduced doses of contrast agents, considering factors such as contrast volume and concentration, injection protocol and advancements in medical technology. The existing body of literature suggests that strategies to reduce ICM administration, while maintaining diagnostic image quality are feasible and potentially beneficial. Studies investigating lower ICM volumes, optimized timing protocols, and the implementation of advanced technologies like dual-energy and photon-counting CT have shown promise in achieving diagnostic accuracy comparable to standard approaches. Moreover, considerations for patient-specific factors, such as body weight, renal function and allergies, play a pivotal role in shaping individualized imaging protocols. The endeavor to reduce ICM is not solely about reducing doses but involves a careful balance between diagnostic accuracy and mitigating potential contrast-induced adverse effects. As medical technology continues to evolve, radiologists, radiographers and healthcare providers must remain attuned to the latest evidence-based guidelines and best practices. In navigating this complex landscape, it is essential for healthcare professionals to exercise clinical judgment, considering the specific clinical context, patient characteristics, and imaging goals. The ultimate goal is to deliver precise and accurate diagnoses while safeguarding patient well-being. As we move forward, collaboration between researchers, radiologists, radiographers and clinicians will contribute to the ongoing refinement of protocols, ensuring that CTPA with reduced ICM dose continues to be a valuable and safe diagnostic tool in the realm of the PA imaging.
None.
None.
None.