Nanocellulose is possessing the unique physical/structural properties, low product cost, renewable, biodegradable, and biocompatible material. For these characteristics, nanocellulose is applicable the many industrial fields such as energy, medical, packing and so on. Nanocellulose from wood and other higher plants is typically isolated as cellulose nanofibers (CNFs) and cellulose nanocrystals (CNCs). Specially, CNFs have strong water absorption capacity and thickening effect, which enhances the viscosity and apply to cosmetic applications. However, CNFs raise the safety concern because they have the high aspect ratio and fibrous morphology. For these reason, this study carried to cytotoxicity, skin irritation test and eye irritation test to find the cosmetic application of CNFs. As a result of this study, CNFs significantly induced cytotoxicity to HaCaT cells (≥ 156 µg/ml) and HDF-α cells (≥ 313 µg/ml). But, CNFs did not induce the skin and eye irritation on 3D models. Taken together, this study suggested that appropriate concentration setting is needed to usage of CNFs as the cosmetic material.
Nanocellulose, Cellulose nanofibers (CNFs), Cytotoxicity, Skin irritation, Eye irritation
Recently, the natural polymers have received extensive extension in many application fields [1]. These natural polymers exist in a variety of natural organisms such as alginate, chitosan, collagen, starch, cellulose and so on [2,3]. The cellulose is the most abundant natural polymer on earth among the natural polymers [4]. Cellulose is considered a substitute for petroleum-based polymer products in many industrial sectors [5].
Also, nanocellulose has attracted rapidly growing scientific and technological interest from academic and industrial researchers [6,7]. Nanocellulose can be defined as cellulose in the form of nanostructures, which are features not exceeding 100 nm at least in one dimension. In other dimensions, these structures can reach hundreds of nm, micrometer, or even more, particularly in the case of eletrospun nanofibers [8]. Nanoscale materials have different properties that offer benefits for energy, medical, and a wide range of consumer goods. Nanocellulose possesses a wide spectrum of advantageous physical, chemical and biological properties. Its large specific surface area enables the adsorption of various atoms, ions and molecules [8]. Nanocellulose materials are five times lighter than iron, but they are high in strength and do not expand even when heated, and most of all, they are environmentally friendly because they get raw materials from plants [9].
The two main types of nanocelluloses are cellulose nanofibers (CNF) and cellulose nanocrystals (CNC). Both CNFs and CNCs are nanoscale cellulose fibers that have shown reinforcing effects in polymer nanocomposites. CNCs and CNFs are different in shape, size and composition [10]. Cellulose nanofiber (CNF) is produced by mechanical treatment with or without enzymatic or chemical pre-treatment. The material consists of long and thin fibers which form a three-dimensional network. It has high viscosity and high water holding capacity. They were able to produce nano-sized fibrils with narrow size distribution [11]. Treating cellulose with sulphuric acid hydrolyzes the amorphous regions. The result is a very highly crystalline material called cellulose nanocrystal (CNC). This fiber is rod-like and stiff. It has a narrow size distribution and is drastically shorter than CFC. CNC has lower viscosity and yield strength than CNF, and it is not as good at holding water. On the other hand, it exhibits self-assembly and birefringence [12]. Although cellulose is considered as non-toxic, nanoscale dimension of nanocellulose may imply different biological effects from convention cellulose [13]. Since different phyco-chmical properties (such as size, shape, surface area and charge) can be played important roles in toxicological aspects [9]. It is important to evaluate and confirm the safety of nanomaterials as the presence of nanocellulose in consumer products increases [14,15]. Nanocellulose has been applied to the field of cosmetics and commercialized. Specially, it is used as an additive in mask packs and basic cosmetics. It is also attracting attention as a thickening agent that maintains a certain viscosity in cosmetics. Consumers nowadays require objective assessments and safety information of cosmetic materials. Thus, the industrial world faces these challenges to satisfy the consumers’ needs, including the rising demand for cosmetics development without animal experiments. For this reason, we investigated the skin and eye toxicity for cellulose nanofibers (CNF) using cell lines, reconstructed human epidermis model and reconstructed human cornea-like epithelial tissue model in this study.
The cellulose nanofibers (CNF) obtained from CNNT (Suwon-si, Gyeonggi-do, Korea), as this company is the Korean company specializing in cellulose nanofibers production and material development. This company provided the characteristic analysis data for this study. The cellulose nanofibers (CNF) were characterized using a scanning electron microscope (JEOL JSM-6700F) and particle size analyzer (Mastersizer 2000, MALVEN, UK) (Table 1 and Figure 1). The test substance, cellulose nanofibers (CNF), was serially diluted with distilled water for treatment.
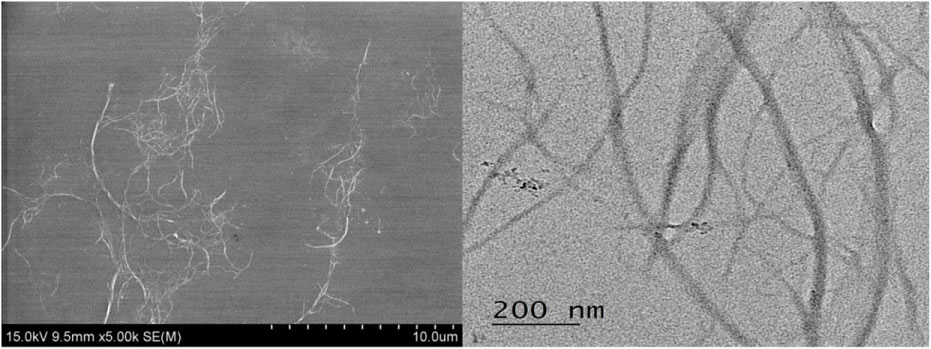 Figure 1: Morphology of the cellulose nanofiber (CNF).
View Figure 1
Figure 1: Morphology of the cellulose nanofiber (CNF).
View Figure 1
Table 1: Characterization data of the cellulose nanofiber (CNF). View Table 1
The human skin keratinocyte cell (HaCaT) was received from the Yonsei University Health System (Seoul, Korea). The human Dermal Fibroblast cell (HDF-α) were received from Biosolution co. Ltd. (Seoul, Korea). HaCaT cells were cultured in Minimum Essential Media (Gibco, USA) and HDF-α cells were cultured in Fibroblast Growth Basal Medium (Lonza, USA). All media were supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories Inc, Logan, UT), 1% antibiotic-antimycotic (100 ×) (Life Technologies Inc., Carlsbad, CA), at 37 ℃ in a humidified atmosphere of air containing 5% CO2.
HaCaT and HDF-A cells were seeded at 1 × 104 cells per well in 96-well plates (Thomas Scientific, Inc., USA) in a humidified atmosphere of 5% CO2 at 37 ℃. All cells were incubated with culture medium for 24 hrs and subsequently treated with various concentrations of cellulose nanofibers (CNF) for 24 hrs. The test substance was diluted in 8 steps using the culture medium and then they were treated in each well. The cell viability was determined by using the 3-(4-5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Corp, St. Louis, MO) assay. MTT solution (5 mg/ml) was added to 96-well plates (200 µl) in each well. The plates were incubated for 3 h at 37 ℃ in a humidified atmosphere of 5% CO2. The supernatants were removed and DMSO was added to each well to dissolve the resultant formazan crystals. The absorbance of each well at 540 nm was measured by using an ELISA reader (Spectra Max M2, Molecular Devices, USA) and then used to calculate the number of viable cells. Cell viability was presented as a percentage relative to the control cells.
LabCyte EPI-MODEL24 was purchased from Japan Tissue Engineering Co., Ltd. (Gamagori City, Japan). It consists of normal human epidermal keratinocytes whose biological origin is neonate foreskin. To stabilize the epidermal keratinocytes model, the tissues were transferred to a 6-well plate containing 1 mL of assay media per well and pre-incubated in 37 ℃, 5% CO2 incubator for 22 ± 2 hours. Then 25 µL of test substance was applied on to the epidermal keratinocytes model. After 15 minutes of exposure, each tissue was rinsed in a washing procedure where DPBS (Dulbecco's Phosphate Buffered Saline; Lonza, USA) squirted ten times with washing bottle and then PBS residue on the tissue was completely wiped off with a cotton bud. The tissues were transferred to new 24-well plate containing 1 mL of fresh assay medium and cultured in a 37 ℃, 5% CO2 incubator for 42 hours. After the post-incubation, MTT (3-4, 5-dimethyl thiazole 2-yl) 2, 5-diphenyltetrazolium bromide) assay was performed to measure the survival rate of the 3D epithelial model. The test material is considered to be irritative to skin if the viability is less than 50% after treatment.
LabCyte Cornea-MODEL24 was purchased from Japan Tissue Engineering Co., Ltd. (Gamagori City, Japan). It was a reconstructed human corneal epithelial tissue produced using normal human corneal epithelial cells. To stabilize the corneal epithelium model, the tissues were transferred to a 6-well plate containing 1 mL of assay media per well and pre-incubated in 37 ℃, 5% CO2 incubator for 22 ± 2 hours. Before the material was treated, 20 µL of DPBS (Dulbecco's Phosphate Buffered Saline; Lonza, USA) was pre-treated for 30 min in 37 ℃, 5% CO2 incubator on the surface of the model to simulate a humid eye condition. 50 µL of the liquid test substance was uniformly applied onto the corneal epithelium model and reacted in 37 ℃, 5% CO2 incubator for 30 minutes. After the reaction, the cornea-like epithelium model was washed with 300 mL DPBS, and the washed specimen was immersed in fresh medium for 12 ± 2 minutes at room temperature. Then, the cells were transferred to a 6-well plate containing 1 mL assay medium per well and cultured in a 37 ℃, 5% CO2 incubator for 2 hours. After the post-incubation, the tissues are each transferred to a well containing the WST-8 (Dojindo Molecular Technologies, Inc., Japan) medium in a 1:10 dilution with Earle balanced salt solution (EBSS). After four-hour WST-8 incubation, the orange water-soluble formazan salt is formed in the WST-8 medium by cellular mitochondria and optical density (OD) of the WST-8 medium is measured using a spectrophotometer at 450 nm and 650 nm as reference. Relative cell viability is calculated for each tissue as % of the mean of the negative control tissues. Test chemicals that produce relative cell viability below 40% of the negative control are predicted to be irritants.
Statistical analyses were performed using SPSS 12.1, and data were expressed as the mean ± standard error (SE). A one-way analysis of variance (ANOVA) was applied to test all the data. A value of p < 0.05 indicated statistical significance.
The cytotoxic effect of cellulose nanofibers (CNF) was evaluated using MTT (3-4, 5-dimethyl thiazole 2-yl) 2,5-diphenyltetrazolium bromide) assay on keratinocyte (HaCaT) cells and fibroblast (HDF-α) at 8 dose levels (39, 78, 156, 313, 625, 1250, 2500, 5000 µg/ml). As result of cytotoxicity test, CNF was significantly induced cell growth inhibition up to 156 µg/ml (HaCaT) and 313 µg/ml (HDF-α) when compared with negative control (p < 0.05) (Figure 2). The positive control (0.5% SDS (Sodium Dodecyl Sulfate)) showed significant cytotoxicity (p < 0.05) when compared with the negative control.
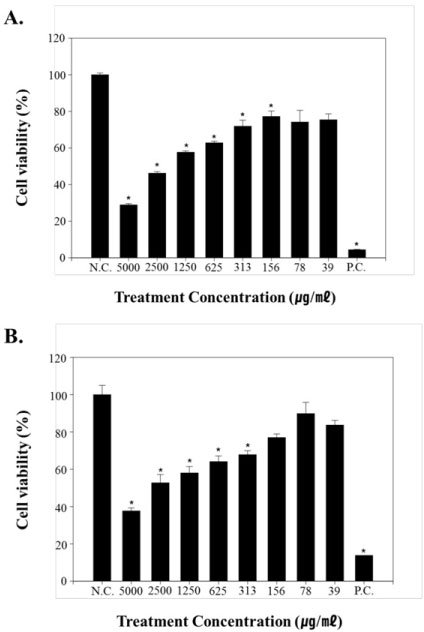 Figure 2: Cytotoxicity evaluation of cellulose nanofibers (CNFs) on; A) HaCaT cells and; B) HDF-α.
View Figure 2
Figure 2: Cytotoxicity evaluation of cellulose nanofibers (CNFs) on; A) HaCaT cells and; B) HDF-α.
View Figure 2
To confirm the applicability as a cosmetic material for cellulose nanofibers (CNF), the skin irritation test and the eye irritation test were carried out using an animal alternative test method. In the irritation test, the cells in the LabCyte EPI-MODEL24 models were exposed to positive chemicals of Ethyl alcohol for 15 minutes and post-incubated for 42 ± 2 hours. The viability was decreased to 13.57 ± 1.39% by SDS. However, the viability was not decreased in the test substance. It was 106.80 ± 5.77% in the cellulose nanofibers (CNF). No significance was found in the CNF treated group in the statistical analysis (Figure 3A). As a result of this test, CNF can be classified as a non-irritant substance in the 3D Epidermal Model. Additionally, It was evaluated the ocular irritancy of CNF using in 3D Reconstructed Human Cornea Model. In the irritation test, the cells in LabCyte Cornea-MODEL24 models were exposed to positive chemicals of 5% sodium dodecyl sulfate (SDS) for 15 minutes and post-incubated for 42 ± 2 hours. The viability was decreased to 8.59 ± 0.02% by SDS. However, the viability was not decreased in the test substance. It was 92.91 ± 7.11% in the cellulose nanofibers (CNF). No significance was found in the CNF treated group in the statistical analysis (Figure 3B). As a result of this test, CNF can be classified as a non-irritant substance in the 3D Cornea Model.
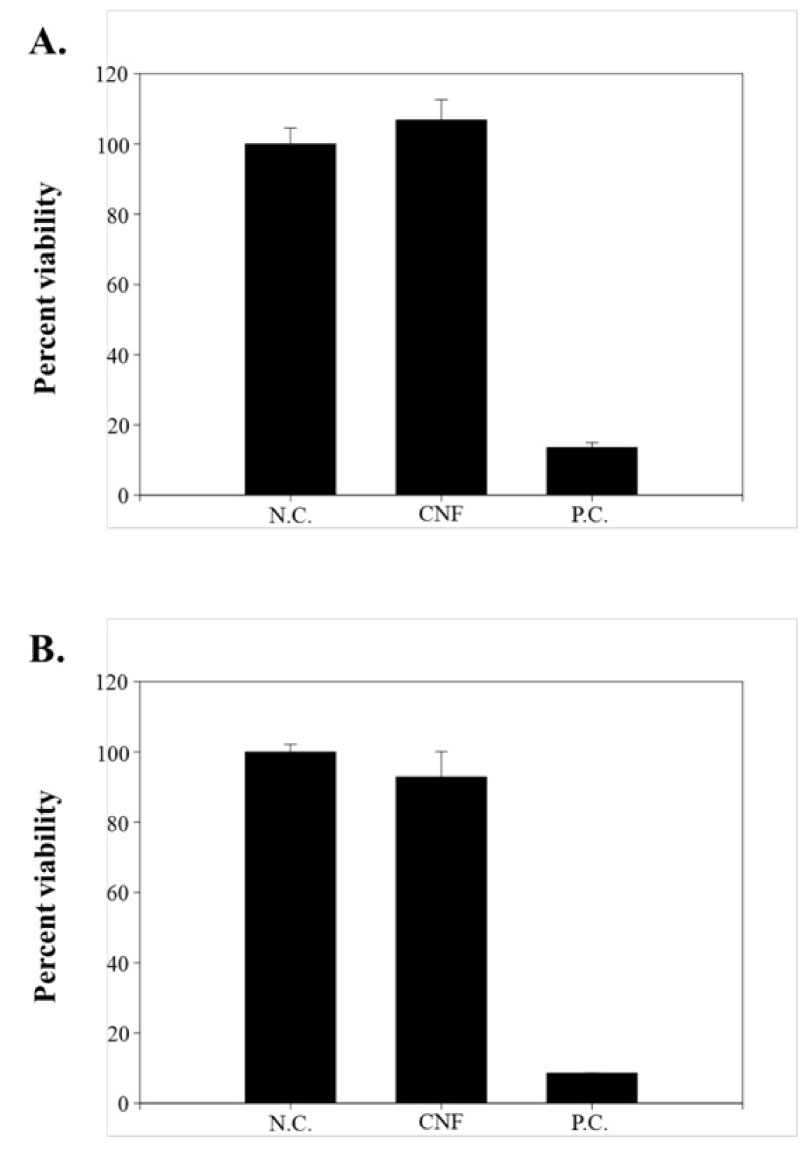 Figure 3: The skin irritation test and the eye irritation test were performed using the; A) 3D Epidermal Model and; B) 3D Reconstructed Human Cornea Model.
View Figure 3
Figure 3: The skin irritation test and the eye irritation test were performed using the; A) 3D Epidermal Model and; B) 3D Reconstructed Human Cornea Model.
View Figure 3
Nanocellulose is considered to be-eco-friendly novel nanomaterials with many beneficial characteristics broadly used in automotive, electronics and appliances, paper and paperboard, food packaging, hygiene and absorbent, medical, cosmetic and pharmaceutical products [14,16-18]. As the growth of Nanocellulose industry, its exposure probility also increases in occupational setting. The main forms of wood-based Nanocelluloses are cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs) [19]. Both forms are extracted from plants via a purification and homogenization pre-treatment step [19]. CNCs are smaller and tend to be stiff, with lengths between 50 ~ 350 nm and widths of 5 ~ 20 nm, while CNFs are flexible with lengths tyrically > 1 µm and widths of 20 ~ 100 nm [19,20]. Nanocelluloses are bio-based, inert materials that may lead to potential to the poorly soluble, low toxicity (PSLT) dust [19]. However, the fiber paradigm highlights the importance of the form, shape and biological interaction of a substance when brought into contact with mammalian cells and tissues [21].
Recently, some researchers have studied about the cell response to CNF exposure. Lopes, et al. [9] studied cytotoxicity, oxidative stress and cytokine secretion by different CNFs in human dermal, lung, and immune cells. In this study, CNF exposure did not induced oxidative stress. However, unmodified CNF elevated pro-inflammatory cytokines level over 250 µg/ml treatment-concentration [22]. Ilves, et al. [23], a similarly designed study, examined the cytotoxicity and pro-inflammatory cytokine production in THP-1 cells following exposure to CNFs [23]. Cytotoxic effects of the unmodified CNFs were observed over 10 µg/ml at 3, 6, and 24 hrs after treatment. Similarly, increased expression and protein production of pro-inflammatory cytokines were observed after 3, 6, and 24 h of exposure, starting at concentrations of 10 µg/ml. Most studies have shown that CNF has a toxic effect on the lung cells.
Unlike these studies, our study focused on the toxic effects of CNF in cosmetic application. Nanocellulose is highly dispersible in water, has high hydrophilicity and high aspect ratio. These properties make it easy to overlap which can sharply change the viscosity and gelation can be achieved at a very low concentration [24]. A thickener is a material that gellifies when making gel-type cosmetics. It enhances the esthetic value of the product by enhancing the durability of the cosmetics and imparting rigidity, smoothness, and soft touch. Carbomer is the most commonly used thickener in cosmetics, but it has low adsorptivity to water, induced blowing of powder and using the benzene as a carcinogen in the synthesis. Cellulose nanofibers (CNFs) have strong water absorption capacity and thickening effect. It is a material obtained from nature, so it is easy to reproduce and it is possible to decompose naturally when disposing. For these reason, CNFs have been attempting to apply to a variety of cosmetic fields. Our study carried out cytotoxicity test, skin irritation test and eye irritation test to confirm the applicability of the cosmetics industry. As a result of cytotoxicity test, CNFs was significantly induced cell growth inhibition up to 156 µg/ml (HaCaT; human skin keratinocyte cells) and 313 µg/ml (HDF-α; human Dermal Fibroblast cells) when compared with negative control (p < 0.05). The skin irritation test and the eye irritation test were performed using the 3D Epidermal Model and 3D Reconstructed Human Cornea Model. However, CNFs did not induce the skin and eye irritation at 5000 µg/ml. In conclusion, the results of the present study showed that the CNFs inhibited the growth of skin related cells but it did not induced skin and eye irritation on 3D human skin and cornea model. Through this study, we confirmed the applicability of CNF to cosmetics field. But, Current study only shows short-term 24-hour exposure results. In future studies of CNFs toxicity, there is a need for long-term skin and eye exposure like real industrial workers and consumer. This study may be provided as the basic data to use CNF as a cosmetic material.
This study was supported by the Technology development Program (S2635636) funded by the Ministry of SMEs and Startups (MSS, Korea).