Budd Chiari syndrome (BCS) provides a unique and complex challenge to transplant surgeons. Single centre data is scare and there is no published data on orthotropic liver transplantation (OLT) for BCS from a single centre in an African setting.
The aim was to retrospectively review all patients with BCS who received an OLT in our unit, comparing survival outcomes with matched liver transplant recipients, identifying the underlying thrombotic pathology and the post-transplant morbidities related to the primary pathology.
This is an observational study, performed retrospectively in the 10-year period ending in 2014. Age, gender and ethnically matched non-Budd Chiari recipients are used as a control group. A Kaplan-Meier survival analysis was performed.
240 OLTs were performed in the study period, 14 were for BCS, an incidence rate of 5.83%.
The 90-day mortality in the BCS was high (14.3%), following that, survival remained 78.6% up to the 8-year mark compared to 75.8% in the control group (p-value 0.96).
OLT in BCS in our setting has higher 90-day mortality than OLT for other indications but the 3 and 5 year survival exceeds OLT for other indications. Our overall survival is on par with large European and US registry studies. There is an ongoing risk for significant thrombosis post OLT despite anticoagulation and therefore a need to follow these patient's long term.
Orthotropic liver transplant, Myeloproliferative disorders, Jak2 mutation
BCS: Budd Chiari Syndrome; IVC: Inferior Vena Cava; MPD: Myeloproliferative Disorders; TIPPS: Transjugular Intrahepatic Porto-systemic shunt; OLT: Orthotropic Liver Transplant; Jak2 Mutation: Janus kinase 2 mutation; MELD: Model for End-stage Liver Disease; INR: International Normalised Ratio
Budd Chiari syndrome (BCS) refers to pathological processes that obstruct hepatic venous outflow, which are primarily thrombotic in nature. Thrombosis with obstruction can involve any level of outflow from the hepatic venules, to the IVC, but most commonly affecting the main hepatic veins and the IVC.
BCS is rare. The exact incidence and prevalence are not well established, with numbers differing markedly geographically. Quoted incidences range from 0.8 per million per year [1], to as high as 100 per million per year in China [1,2]. South Africa is considered as a high incidence area [3,4].
BCS is a complex disease with variable presentations including, but not limited to, the classic triad of abdominal pain, ascites and hepatomegaly. Patients can be asymptomatic, present with chronic liver dysfunction and cirrhosis or in 5% of cases, with fulminant liver failure [5]. Other symptoms may include those of the underlying prothrombotic condition or symptoms of the resultant portal hypertension. The etiology of BCS seems to vary geographically. In Asia and historically in South Africa, it is more commonly due to membranous obstruction of the IVC, while in Europe and North America it is due to a diverse group of prothrombotic conditions [5]. Along with inherited disorders such as factor V Leiden mutations, the myeloproliferative disorders (MPD) account for the majority of these prothrombotic conditions. The identification, in 2005, of the Janus kinase 2 mutations has greatly increased the diagnosis of these latent MPD's [6]. The presence of an underlying latent MPD may predict thrombotic complications post-transplantation, hence the importance of making a diagnosis [7].
Untreated, BCS has a progressive course with a poor prognosis. The management of BCS encompasses medical, radiological and surgical modalities. The initial objectives being the prevention of clot progression and the management of ascites and other sequelae of portal hypertension, followed by restoration of hepatic outflow. Angioplasty and porto-systemic shunting, commonly with a TIPS procedure or a surgical shunt, are well established treatment modalities. However, there are no international guidelines or universally adopted algorithms in the management of BCS, with most centers recommending a step-wise and individualized approach.
The role of orthotropic liver transplantation (OLT) in BCS is well established. OLT is the treatment of choice in fulminant BCS and in cases where medical therapy and shunting have failed [2,5,8]. In the non-acute setting, selection for transplantation includes, amongst other criteria, assessment of anticipated long-term survival, and the rapidity of progression. 10-20% of BCS patients come to transplantation [5].
The aim of our study was to retrospectively review all patients with the BCS who received an OLT in the 10-year period between 2004 and 2013 with the primary objective of comparing survival outcomes with other liver transplant recipients from the same unit. In addition, we wanted to identify post-transplantation morbidities, specifically those relating to the underlying thrombotic conditions, to describe the spectrum of these conditions and the association, if any, with the jak2 mutation.
This was an observational study, performed retrospectively. All patients with the diagnosis of BCS who received an OLT at the Wits Transplant Unit from the start of the liver program in 2004 till the end of 2013 were included.
All the information was obtained from the transplant register, the electronic database and the follow up records.
Age and gender matched non-Budd Chiari recipients were used as a control group. Ethics approval was obtained from the University of Witwatersrand Human Research Ethics committee, which adheres to the Singapore Statement.
All data was captured into an excel template. Comparisons between the BCS and control groups used the Chi-squared test for categorical data and the t-test for continuous data.
A Kaplan-Meier survival analysis was completed. All statistical analysis was performed on Microsoft®Excel® (version 14.0.0) and R® (version 3.1.2).
Of the 240 OLTs performed in the study period, 14 were for BCS (n = 14), with an incidence rate of 5.83%.
28 controls were selected and they statistically matched the BCS cohort on age and gender with p values of 0.96 and 0.65 respectively. There were more Caucasians than other races in both groups (Table 1). The reason that Caucasians are the dominant group number-wise likely relates to them having greater access to medical insurance funding in our practice setting.
Table 1: Comparing demographics and characteristics and mortality of study and control groups. View Table 1
A much higher proportion of the BCS group had a MELD (model for end-stage liver disease) scores over 20 compared to the control group, 57.1% versus 35.7% respectively (Table 1).
The etiologies of the underlying thrombotic conditions in the BCS group were identified in 8 patients, with one patient confirmed to have 2 conditions. The reminders were labelled as idiopathic (Figure 1). Two of these diagnoses were made post-transplantation. Only 3 patients had a proven MPD. 9 BCS patients were tested for the JAK2 mutation, with 3 testing positive.
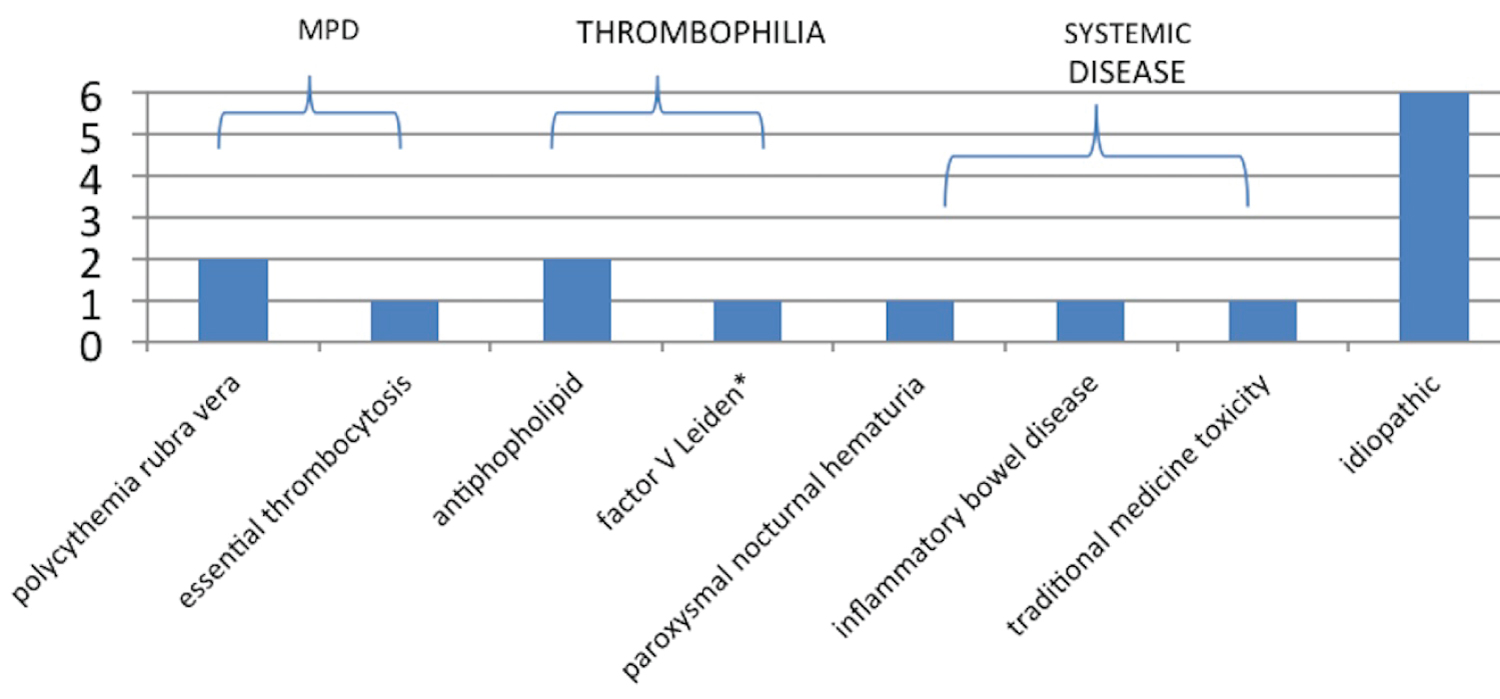 Figure 1: The frequencies of the underlying thrombotic conditions in the BCS group.
View Figure 1
Figure 1: The frequencies of the underlying thrombotic conditions in the BCS group.
View Figure 1
Two patients in the study group developed thrombotic events post-transplantation during the study follow-up period despite being on warfarin. One patient suffered from a thrombotic stroke, the other from a fatal pulmonary embolus. The other 2 mortalities in the BCS group were due to primary graft failure and sepsis.
The mortalities in the control group were due to 2 cases of rejection, 1 myocardial infarct, 1 lymphoma, 1 disseminated aspergillosis and one from recurrent intra-abdominal sepsis.
All the BCS mortalities were in the first 4 months post-transplantation. Following that the survival remained 78.6% up to the 8-year mark. In the control group the 1 and 3 year survival was 85.7% and 75.8% respectively. Thereafter it remained static. Overall survival for the 2 groups at 5 years was the same (p-value of 0.96) (Figure 2).
 Figure 2: The Kaplan-Meier Survival Analysis.
Figure 2: The Kaplan-Meier Survival Analysis.
Overall survival for the BCS group and the control group was not statistically significant with a p-value of 0.96.
View Figure 2
Liver transplantation for BCS was initially performed successfully in 1974 [9]. Because of the rarity of the disease, single center reports of liver transplantation in BCS are small. The largest cohort from a single center found in the literature comes from Germany by Ulrich, et al. [10]. Over a period of 18 years, they performed 42 orthotropic liver transplantations (OLT) in 39 patients with BCS.
Liver transplantation in BCS is technically more challenging. The presence of a large caudate lobe, peri-hepatic and retroperitoneal fibrosis and anti-coagulation, all lend to a more difficult procedure with a higher rate of perioperative blood loss [2]. However, patients' survival post-transplantation for BCS has been documented to be similar to other OLT recipients. 5-year survival is quoted to be between 75 to 89% [10-13].
The frequency of BCS in liver transplant recipients in our institute (5.83%) is high. Similar studies quote incidences ranging from 1% [14], 2.1% [10] to 3.5% [11]. This might infer a higher incidence of BCS in our population. However, the population that presents to our center with liver disease is not a reflection of our general population. Because of the different inclusion criteria for transplantation for BCS depending on the center and country, comparisons of incidence cannot be made.
There is not one set of criteria that is widely accepted for transplantation in BCS. The use of several prognostic tools, such as the Childs-Turcotte-Pugh, the MELD, the clinical presentation, and in some centers, liver biopsy results, makes comparisons between different studies difficult. Our study did not specifically evaluate the indication for enlisting but the MELD score was used as part of the assessment. In both the BCS and control groups, all except 4 patients had MELD scores over 10. The 4 that had low MELD scores were transplanted because they had complications that over-rode their MELD score. The MELD is a well validated score for end stage liver disease and is used widely in liver transplantation programs. The higher MELD score in our BCS group compared to the control group suggest that the BCS group had more advanced liver dysfunction.
However, one criticism of the MELD score in assessing BCS is the use of the INR. As most patients with BCS are on anticoagulation pre-transplantation, their MELD scores are usually falsely elevated. The capping of the INR at 2.5 in BCS has been used in other studies but has yet to be universally adopted [2]. The Rotterdam BCS score that incorporates more clinical features such as ascites and encephalopathy has been validated as a better prognostic score in BCS but has not yet replaced the MELD in assessment of end stage liver disease during the enlisting process for transplantation [15].
42% of our BCS cohort was labelled idiopathic. Most studies report around 20% of their BCS patients as idiopathic [1,16]. There were no reported IVC webs, which may be due to under diagnosing and may account for the higher number of idiopathic BCS in our study. However, it seems more likely that the incidence of webs is much lower in our population than previously reported [4] with our underlying etiology similar to the Europe. 9 of the 14 patients were Caucasian, which supports this statement. As only a small percentage of BCS patients come to transplantation, inferences of the underlying etiologies in the population as a whole cannot be made.
An insufficient number of the BCS patients were tested for the JAK2 mutation. Therefore, we cannot comment on the importance of JAK2 in the prediction of post OLT complications. However, in those that were tested only 3 were positive suggesting that, in our population, it is not a substitute for bone marrow analysis. A follow up study to test for the mutation in the explanted liver specimens might provide more clarity.
BCS patients post OLT remains at risk for thrombotic complications. 2 patients developed significant thrombotic related complications within 2 years despite being on anticoagulation. However unlike most other studies, we have no reports of recurrent BCS. Rates of recurrent BCS vary significantly in the literature ranging from 2-11% [10,13]. Cruz, et al. reported the highest recurrence rate; 3 cases of recurrent BCS out of a total of 11 cases (27%) [14]. Most reported recurrences are within 2 years. Our follow-up period extended beyond this time frame.
The 90-day mortality in the BCS group was 14.3% and in the control group was nearly half at 7.14%. The BCS group survival compared to our control survival at 1 was 78.6% versus 85.7% and at 3 years was 78.6% versus 75.8% respectively. Survival probability did not change at 5 years with a higher overall survival in the BCS group. This difference was not statistically significant (p = 0.99).
The survival of BCS patients in our study is comparable to the 2 largest registry studies in the literature. Our 1 year survival in relation to the European registry and the US registry were 78.6% vs. 76% and 82% respectively; the 3 year survival was 78.6% vs. 76% in the US study; with the 5 year survival being 78.6% vs. 72% in the European study [12,13].
In comparison to the largest single center study from Germany by Ulrich, et al. our survival was lower [10]. Demographically our BCS cohort was comparable to theirs but the use of the Childs-Turcotte-Pugh score as opposed to the MELD score made further comparisons regarding the pre-transplant severity of disease not possible. Their routine use of liver biopsies pre-transplantation may have influenced earlier selection, as evidenced by the fact that 69% of their patients were Childs-Pugh A or B.
A limitation of our study was the small number of patients. Given the rarity of BCS, most single center studies average numbers between 9 and 39 patients. There has yet been no meta-analysis comparing survival of BCS and the largest studies have been registry based.
In conclusion, OLT in BCS in our setting has a higher 90 mortality than OLT for other indications but the 3 and 5 year survival exceeds OLT for other indications. Our overall survival is on par with large European and US registry studies. There is an ongoing risk for significant thrombosis post OLT despite anticoagulation and therefore a need to follow these patient's long term. IVC membranes, which were previously thought to be the main etiology of BCS in South Africa, have not been seen in any of our patients. The underlying pathologies in our study being pro-thrombotic conditions either inherited or acquired.
We have no actual or potential conflict of interests or financial disclosures.