Over six million cases of pneumonia are diagnosed annually in the United States. Clinicians commonly experience uncertainty regarding the accuracy of laboratory tests as these values are not well-published or easily accessible. Performance data of diagnostic tests are needed to assist clinicians in procuring a microbiologic diagnosis.
We undertook a literature search to assess the accuracy of diagnostic tests for pneumonia, identified through a search of MEDLINE-indexed journals. Sensitivity and specificity of diagnostic tests for pneumonia were calculated with respect to various reference standards.
A battery of diagnostic testing is adequate to rule out most typical and atypical bacteria and bacteria. Testing is inadequate to exclude, and empiric treatment should be considered, for clinical suspicion of Nocardia, Rhodococcus, Actinomyces, anaerobic bacteria, mycobacterial infection, and zoonotic-associated bacteria.
The accuracy of any single diagnostic test for pneumonia is generally inadequate to rule out a pathogen. Multiple diagnostic methods are often needed to confidently establish a microbiologic diagnosis. The presence of many pathogens cannot be effectively excluded with current diagnostic testing, and empiric treatment should be considered when there is clinical suspicion despite negative testing.
Pneumonia, Diagnosis, Accuracy
Clinicians should be familiar with the degree of accuracy of laboratory tests in diagnosing pneumonia, especially for those needed to diagnose the immunosuppressed patient. Empiric antibiotic therapy often results in the resolution of symptoms, but microbiologic or serologic confirmation of the pathogen may be necessary for patients who fail to improve. Clinicians face uncertainty regarding the accuracy of diagnostic tests as a result of a lack of concise guidance from professional societies. Too much confidence by the clinician in a negative laboratory test may create false security. A stepwise approach to a diagnosis of pneumonia may reduce diagnostic uncertainty.
We will review the literature and discuss the accuracy of diagnostic tests for infectious pneumonia, starting with, "typical" bacterial pneumonia and zoonotic pneumonia in part 1, fungal pneumonia in part 2, and viral and parasitic pneumonia in part 3 (Figure 1).
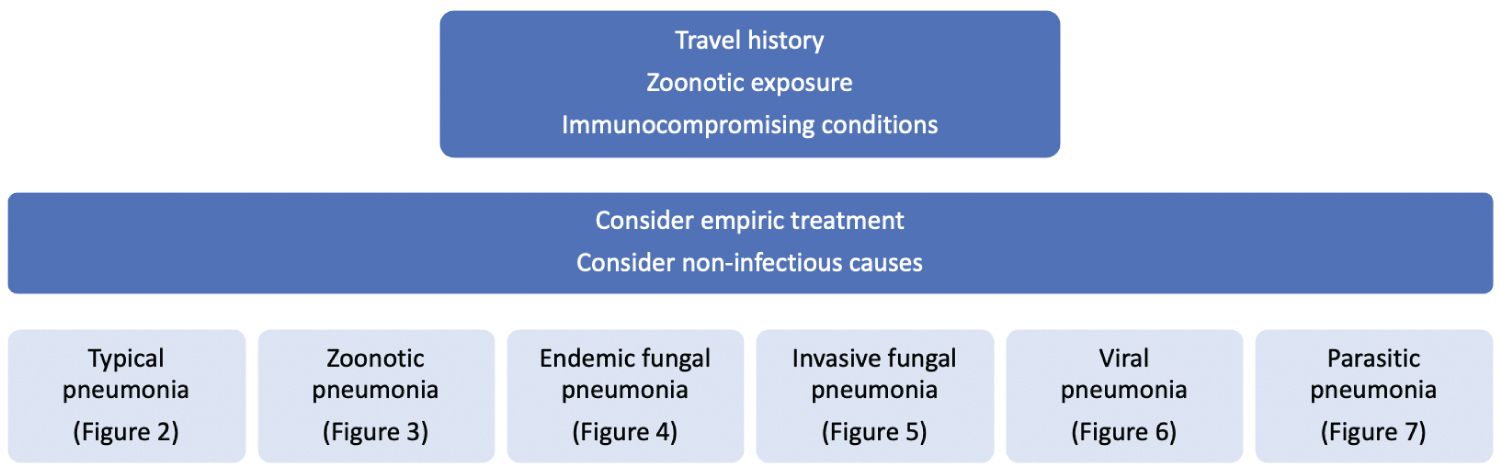 Figure 1: Evaluation of pneumonia. View Figure 1
Figure 1: Evaluation of pneumonia. View Figure 1
Aerobic bacteria comprise the majority of community- or hospital-acquired pneumonia pathogens. Commonly encountered pathogens include Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.
Body fluid or tissue culture is the diagnostic gold standard for most typical bacterial pathogens. The sensitivity of bronchoalveolar lavage fluid (BALF) culture for autopsy-proven cases of community-acquired pneumonia (CAP), utilizing a low threshold of greater than 1,000 colony forming units per milliliter (CFU/mL), has been reported at 56% [1] and 88% [2]. Autopsy studies are at risk of underestimating culture sensitivity for cases in which there is a poor ability to culture those pathogens, but can also overestimate sensitivity in cohorts of more severe pneumonia with a greater bacterial load. Among cases of clinically diagnosed pneumonia, however, the sensitivity is similar and has been reported at 80% [3], 83% [4], and 90% [2]. The use of clinical diagnosis reference standard risks underestimating the true sensitivity of culture, as a case of clinically diagnosed pneumonia may in fact be non-infectious. If the culture threshold is increased to greater than 10,000 CFU/mL, the sensitivity has been reported to reduce to 34% [5], 42% [6], 69% [7], and 85% [2], and is further reduced at a threshold of 100,000 CFU/mL to 16% [5] and 55% [2]. For specimens obtained from patients with clinically diagnosed pneumonia who are treated with antibiotics at the time of culture (cultures that are often obtained due to empiric treatment failure), the reported sensitivity is reduced to 46% [8] and 78% [2] at a threshold of 1,000 CFU/mL, and to a figure as low as 1% [9] and 37% [8] at a threshold of 10,000 CFU/mL. Newer techniques utilizing metagenomic next-generation sequencing (mNGS) have raised doubt into the sensitivity of culture. Among cases of pneumonia with a positive organism on mNGS, BALF culture sensitivity has been reported at only 12% [10], 42% [11], 53% [12], and 67% [13]. These tests may be complementary, however, as mNGS sensitivity has been reported at 30% [10] and 96% [12] of culture-positive cases. The sensitivity of bronchoscopic guided protected specimen brush (PSB) culture for patients with clinically diagnosed pneumonia has been reported at 72% [14] utilizing a threshold of 10,000 CFU/mL. Its sensitivity is similar to that of BALF, but carries an increased risk of complications and has little additional utility. The specificity of culture has been reported at 28% [1], 97% [2], and 100% [5], with the lower end reduced due to colonization of the airways in the absence of pneumonia.
Culturing induced sputum (IS) is less invasive than obtaining specimens by bronchoscopy, and has been reported to be concordant with BALF in 42% [7] and 94% [15] of cases, and with sterile specimen sites such as the pleural space in 96% [16]. Among patients with clinically diagnosed pneumonia, the sensitivity of a good-quality sputum culture has been reported at 35% [16], 38% [3,7], 50% [17], and 82% [6]. BALF culture appears to be superior to that of IS, and the addition of BALF to IS can add an additional sensitivity of up to 36% [7]. Therefore, a culture-negative IS sample should still prompt consideration of bronchoscopy, even in instances of concurrent antibiotic usage.
IS gram stain sensitivity varies by bacterium, and has little value in adjusting antibiotics. Among cases of culture-positive pneumonia, the pooled sensitivity of a sputum gram stain for S. pneumoniae has been reported at only 59% [18], with a specificity of 87% [18]. The pooled sensitivity of a gram stain for H. influenzae has been reported at 78% [18], with a specificity of 96% [18]. The sensitivity of a gram stain for S. aureus has been reported at 72% [18], with a specificity of 99% [18]. Lastly, the sensitivity of a gram stain for gram-negative bacilli has been reported at 64% [18], with a specificity of 99% [18].
BALF culture for patients with hospital-associated pneumonia (HAP) or ventilator-associated pneumonia (VAP) possesses similar accuracy to those with CAP, validating its diagnostic accuracy. Among patients with either histopathologic-proven HAP or VAP, the pooled sensitivity of BALF culture utilizing a threshold of 10,000 CFU/mL has been reported at 71% [19], with a specificity of 80% [19]. As is true with CAP, specificity is poor owing to airway colonization so higher thresholds are generally used to improve specificity. An endotracheal aspirate culture of greater than 100,000 CFU/mL for patients with histopathologic-proven HAP or VAP has a pooled sensitivity similar to BALF, reported at 76% [19], with a specificity of 68% [19]. Similarly, the specificity of culture is reduced due to bacterial colonization of the trachea, which is common among patients who require intubation. PSB culture in histopathologic-proven VAP has a sensitivity similar to that of BALF or endotracheal aspirate with a pooled sensitivity utilizing a threshold of 1,000 CFU/mL of 61% [19], but contributes no additional yield to that of BALF. PSB is not commonly used, but has good microbiological correlation with BALF [20]. In contrast, endotracheal aspirate culture has poor concordance with either BALF or PSB, and may fail to identify pathogens detected by more invasive methods [20].
The urine Streptococcus antigen (UAT) immunochromatography test can be used to detect pneumonia caused by S. pneumoniae. The pooled sensitivity of the UAT among culture-positive pneumococcal pneumonia has been reported at 74% [21] and 75% [22], with a specificity of 95% [22] and 97% [21], and is consistent in patients both with and without immunocompromising conditions [23,24]. The addition of the Streptococcus UAT to culture contributes up to an additional sensitivity of 20% [25] and 50% [26]. The Streptococcus antigen can be detected in BALF but has not been effectively compared to urine samples. In cases of culture-positive pneumococcal disease, BALF antigen sensitivity has been reported at 50% [27] and 95% [28], with a specificity of 87% [28], and like the UAT may be positive in culture-negative infection [27]. Whether there is additional value of obtaining the BALF Streptococcus antigen to the UAT is uncertain and the BALF antigen might be best reserved for anuric patients.
The multiplex bacterial PCR (mPCR) can be used to rapidly identify pathogens in either BALF or IS. Among culture-positive cases, mPCR sensitivity for S. pneumoniae has been reported at 86% [29], with a specificity of 81% [29]. Similarly, the sensitivity of the bacterial mPCR for H. influenzae has been reported at 88% [29], with a specificity of 64% [29]. Among all culture-positive cases of pneumonia, the sensitivity has been reported at 67% [30,31] and 68% [32], with specificity of 86% [32] and 99% [30]. Lastly, among all cases of clinically diagnosed pneumonia, the sensitivity of BALF mPCR detection of all typical bacterial pathogens has been reported at a similar value of 66% [33]. Despite its limitations, mPCR can provide a reported additional sensitivity of 14% [29] and 50% [30] to culture for pathogens such as H. influenzae and S. pneumoniae, but these are generally covered with empiric antibiotics. mPCR has been used to detect more resistant bacteria including P. aeruginosa, E. coli, Enterobacter cloacae, and K. pneumoniae in instances of culture-negative samples, and the addition of mPCR to culture and cytology has been reported to increase the sensitivity of detection by nearly an additional 50% [33]. Therefore, the addition of mPCR should be considered in cases of culture-negative pneumonia or when a more rapid diagnosis is needed.
Legionella species are aerobic, gram-negative intracellular bacteria transmitted through aerosolized droplets of contaminated water, often leading to pneumonia with extrapulmonary manifestations.
BALF culture sensitivity in clinically diagnosed cases of legionellosis has been reported at 65% [34]. As a clinical diagnosis can be challenging, the sensitivity of Legionella culture risks overestimation. Of PCR-positive cases of Legionella, interestingly, culture sensitivity has been reported to be similar at 45% [35] and 76% [34]. If extending the reference cohort to include cases detected by either UAT, DFA, or PCR, the sensitivity reduces slightly to 43% [36] and 51% [37]. Culture may provide additional sensitivity to PCR and UAT, but requiring nearly five days to perform, its utility is limited. The specificity of culture approaches 100% [34], as colonization is not known to occur, and a positive culture should be considered to be infectious.
Direct fluorescent antigen (DFA) detection of Legionella can be performed on BALF. Among culture-positive cases of legionellosis, however, the sensitivity has been reported at only 67% [38], with a specificity of 100% [38]. Expanding the reference standard to include either culture, UAT, IF, or PCR reduces the sensitivity of DFA even more to a reported 19% [36]. DFA is not known to provide additional sensitivity to that of culture or PCR and for this reason, although commercially available, DFA is not generally recommended.
Serological methods can be used to diagnose Legionella infection utilizing either IF or ELISA. IF sensitivity has been reported at 71% [39] of UAT-positive cases with a specificity of 66% [39], but paradoxically the sensitivity has been reported to increase to 94% [36] of cases positive by either culture, UAT, DFA, or PCR. IF titers may be elevated in cases not detected by either culture or UAT [36,39], although it is not certain if IF is able to detect PCR-negative cases. ELISA serology sensitivity has been reported at 63% [39] of UAT-positive cases, and like IF, ELISA can detect UAT-negative infection. The addition of ELISA to culture has been reported to increase sensitivity by 24% [40], while the addition of ELISA to PCR has been reported to increase the sensitivity by only 6% [40]. Both IF and ELISA are commercially available, and as the addition of serology improves sensitivity to any individual diagnostic test, either should be considered in the initial diagnosis.
The L. pneumophila serotype 1 antigen can be obtained from either urine or BALF. Among cases diagnosed by either culture, serology, or PCR, the sensitivity has been reported at74% [41], and of cases positive by either culture or serology the sensitivity increases to 91% and 97% [42], illustrating the added value of PCR. The pooled sensitivity of the UAT has been reported at 77% [43] of PCR-positive cases, and at 87% [35] and 96% [44] of culture-positive cases. The existence of non-pneumophila serotypes limits the sensitivity of the UAT in diagnosing Legionella infection. The specificity of the UAT is excellent, having been reported at 95% [35], 99% [41], and 100% [43]. The L. pneumophila antigen can also be detected in BALF, but has been poorly studied [45], and like the pneumococcal antigen should be reserved for anuric patients. The UAT has satisfactory sensitivity to replace culture as an initial diagnostic test, but when used alone lacks the accuracy to be utilized without subsequent testing such as serology or PCR.
PCR is the most accurate test for the diagnosis of legionellosis. Among culture-positive cases, the pooled sensitivity of Legionella PCR has been reported at 83% [46], weighted downward by a single outlying study, with a specificity of 90% [46]. Of cases positive by culture, UAT, or a four-fold increase in serologic titer rise, the pooled sensitivity has been reported to as high as 97% [43], with a specificity of 99% [43]. Unlike BALF PCR, serum PCR sensitivity is poor, having been reported at 30% [47], 35% [36], and 43% [48] of culture-positive or serologically-diagnosed cases, and lacks the accuracy to recommend its use. Although BALF PCR is the most sensitive laboratory test, the addition of either UAT, culture, or serology can increase the sensitivity of PCR alone by up to 16% [40,44], and thus multiple diagnostic methods should be considered.
Mycoplasma pneumoniae is an intracellular bacterium acquired through inhalation of respiratory secretions, often leading to pneumonia with extrapulmonary manifestations.
The sensitivity of culture in serologically diagnosed cases has been reported at 13% [49] and 70% [50], and among PCR-positive cases has been reported at 13% [49], 58% [51], 61% [52], and 90% [53], with a specificity approaching 100% [50,51]. Mycoplasma culture may require up to three weeks to perform and fails to provide additional cases to those made by a combination of serologic techniques [50,51], and is thus not generally recommended [54].
Rapid antigen immunochromatographic testing has a sensitivity reported at 63% [49] of PCR-positive cases, with a specificity of 91% [49]. With the widespread availability and superior accuracy of PCR, rapid testing is neither commercially available nor recommended.
Serology is an effective means of diagnosing Mycoplasma, as a four-fold rise in antibodies between the acute and convalescent phase is diagnostic. Among clinically diagnosed cases, the sensitivity of a baseline ELISA IgM has been reported at 81% [55]. This value may overestimate a challenging clinical diagnosis. The sensitivity indeed is reduced in PCR-positive cases, reported at 16% to 42% [56] and 65% [57], and likewise in patients confirmed by seroconversion has been reported at 32% to 84% [56]. ELISA IgM sensitivity approximately doubles from the acute phase to the convalescent phase [56], and therefore obtaining serial serology is recommended. Owing to a weak IgM response, adults have a sensitivity less than that of children, whose sensitivity among cases with either seroconversion or a rise in IgG approaches 80% [53,58]. ELISA IgG sensitivity in the acute phase of PCR-positive cases has been reported at only 37% to 89% [56], but approaches 100% [56] in the convalescent phase, with a specificity of 63% [55], 88% [56], and 100% [59]. CF is an older serologic technique that is not now commercially available. CF sensitivity in PCR-positive cases has been reported at 65% [56] and 100% [51], and is similar among culture-positive cases at 90% [60], with a specificity of 94% [60] and 97% [56]. CF antibodies are often undetectable in the acute phase, but the sensitivity approaches 100% [51] within two weeks. Microparticle agglutination assay (MAG) sensitivity in serologically-confirmed cases has been reported at 87% [59], but MAG is also not commercially available and not recommended due to its modest accuracy. IF appears to be even less accurate. Among CF-positive cases, the sensitivity of IF for IgM and IgA has been reported at 76% [50] and 100% [50], respectively. Only ELISA serology is commercially available, and to optimize sensitivity ELISA should be repeated at three to four weeks if both baseline serology and PCR are negative.
The pooled sensitivity of Mycoplasma PCR has been reported at only 62% [61] of serologically diagnosed cases, illustrating the value of combining serology and PCR to maximize yield. Among clinically diagnosed cases, the sensitivity has been reported at only 21% [55] and 50% [49], with a specificity reported at 94% [62], 96% [61], and 98% [55], but the low sensitivity may be a result of clinical overdiagnosis. PCR can effectively replace time-consuming culture, with sensitivity reported at 100% [63] of culture-positive cases. The viral mPCR positive predictive agreement (PPA) with Mycoplasma PCR has been reported at 78% [64], 88% [65], and 96% [66], with a negative predictive agreement (NPA) of 100% [65,67]. This suboptimal agreement only further reduces an already modest sensitivity. Although PCR results can be rapidly obtained, its limited sensitivity means it should be supplemented with serology.
Less commonly used diagnostic techniques, including nuclear acid sequence-based amplification (NASBA) and loop-mediated isothermal amplification (LAMP) have been used as alternatives to PCR. The pooled sensitivity of NASBA has been reported at 77% [67] of PCR-positive cases, with a specificity of 98% [67]. LAMP sensitivity has been reported at 90% [68] of PCR-positive cases, with a specificity of 98% [68]. Neither of these methods is commercially available nor does either appear to add value to the combination of PCR and serology.
Chlamydophila pneumoniae is an obligate intracellular bacterium that can lead to community acquired pneumonia with extrapulmonary manifestations, although often presenting with more chronic symptoms than Mycoplasma.
BALF culture sensitivity has been reported as low as 0% [55] of ELISA-positive cases, 8% [69] of cases positive by PCR or serology, and 64% [70] and 88% [71] of cases positive by DFA or PCR. When culture and DFA are used in combination, the sensitivity approaches that of PCR [71], however with the availability of rapid PCR these methods may be less attractive. Specificity is excellent, having been reported at 97% [71] and 100% [70], but culture requires several weeks and is not commercially available, making the method of limited diagnostic utility [54].
Similar to Mycoplasma, serology can be an effective means to diagnose Chlamydophila infection. ELISA serology sensitivity has been reported at 36% [72], 89% [73], 93% [70], and 100% [69] of cases positive by either DFA, culture, or PCR, with a specificity of 95% [72,73]. CF is no longer widely commercially available, but the sensitivity has been reported at 69% [73] of cases with two positive confirmatory serologic tests, with a specificity of 99% [73], providing little value to more rapid serologic techniques. IF sensitivity has been reported at 97% [74] of clinically diagnosed cases, with a specificity of 77% [74], although this may be an overestimation as the diagnosis is challenging. The sensitivity among PCR-positive cases has been reported widely at 32% [72] and 100% [69], and of cases detected by ELISA or CF, has been reported at 88% [73] with a specificity of 99% [73]. Unlike ELISA or CF, IF is commercially available and should be used concurrently with PCR.
Among culture-positive cases, the sensitivity of PCR from nasopharyngeal or oropharyngeal swabs has been reported at 90% [75] and among clinically diagnosed cases reported at 71% [74]. The sensitivity has further been reported at 75% [76] of serologically diagnosed cases by IF, 39% [72] and 75% [77] of cases positive by either culture or serology, and 76% [77] of cases positive by either culture or DFA. When used in conjunction with serology, PCR appears to be suitable to replace time-consuming culture. The specificity of PCR has been reported at 84% [72] and 99% [77,78], reduced due to detection of true-positive cases not identified by serology or culture. Viral mPCR PPA with Chlamydophila PCR has been reported at 40% [65] and 100% [66], with an NPA of 100% [65,66], although it has been evaluated in very few cases. PCR is widely available and although results can be rapidly obtained, due to its limited sensitivity should be supplemented with serial serologic testing.
Anaerobic bacteria, including Actinomyces spp, Prevotella spp, Bacteroides spp, Peptostreptococcus spp, Veillonella spp, and Fusobacterium spp lead to lung infection more indolent than those caused by aerobic bacteria, commonly manifesting as pulmonary cavitation or pleural space infection. Similar opportunistic bacteria, such as Nocardia asteroides and Rhodococcus equi are included in this group.
Anaerobic bacteria have historically been recovered poorly in culture. BALF anaerobic bacteria culture sensitivity has been reported at 43% [79] of clinically diagnosed cases, which may be somewhat underestimated as Mycobacterium tuberculosis, lymphoma, endemic fungal infection, or cryptogenic organizing pneumonia may mimic anaerobic infection. BALF anaerobic culture sensitivity has been reported as low as 1% [79] of cases with a positive concurrent pleural culture, and among PCR-positive cases only 22% [80] and 32% [81]. These values are consistent with pleura culture sensitivity in cases of rRNA-positive empyema, being less than 10% [82]. The sensitivity of BALF culture is poor as most anaerobic lung infections develop cavities that sequester from the airways. The specificity is uncertain, but similar to most bacterial pneumonia, is suspected to be very low, or even worse, due to airway colonization. Actinomyces culture sensitivity among histopathologic-proven cases has been reported in line with other anaerobic bacteria at 8% [83] and 50% [84]. Specificity likewise is poor as airway colonization is common [84]. Nocardia culture sensitivity has been reported at 77% [85] of clinically diagnosed cases, but among PCR-positive cases of nocardiosis, the sensitivity reduces to 62% [86]. Although reported in only small numbers, the sensitivity has been reported at 100% [10] of cases detected by mNGS. Rhodococcus culture sensitivity has been reported at 57% [87] of clinically diagnosed cases, although few studies exist to verify the accuracy. Empiric treatment should be considered for anaerobic pneumonia, even with negative cultures, as the sensitivity of culture is poor.
Nocardia PCR sensitivity in respiratory secretions has been reported at 88% [86] and 100% [88] of culture-positive cases, with a specificity of 74% [86] and 100% [88], the reduced specificity likely indicating the ability of PCR to detect culture-negative cases. Colonization may occur, however, and PCR-positive specimens have indeed been identified in asymptomatic, culture-negative surveillance bronchoscopy [89]. PCR sensitivity for Rhodococcus has been reported at 90% [87] of culture-positive cases, with a specificity of 81% [87]. Although PCR may be performed on BALF to detect Actinomyces, Nocardia, and Rhodococcus, none of these tests are widely commercially available. Due to the poor sensitivity of BALF culture, supplementation with PCR should ideally be considered when there is clinical suspicion.
Mycobacterium tuberculosis (MTB) is an aerobic acid-fast bacterium (AFB) acquired through aerosolization of infected secretions. Nontuberculous mycobacteria (NTM), most commonly encountered M. avium and M. intracellulare, are genomically similar bacteria most commonly acquired through soil or aerosolized water exposure.
BALF or IS AFB smear is commonly used as a rapid test to detect the presence of MTB. Among patients with culture-positive MTB, AFB smear sensitivity has been reported at 47% [90] and 93% [91], and among clinically diagnosed infections reported at 80% [92] and 83% [92], with a specificity of 97% [91]. Multiple smears do not appear to significantly increase the sensitivity over a single morning smear. IS or expectorated sputum sensitivity is slightly less than BALF sensitivity and among culture-positive cases has been reported at 39% [90]. Among cases of culture-positive NTM pneumonia, AFB smear sensitivity has been reported at 50% [93], 53% [94], and 66% [95].
BALF MTB culture sensitivity among clinically diagnosed infection has been reported in a wide range at 5% [96], 20% [97], 28% [98], 47% [99], 56% [100], and 73% [101]. These estimates are likely accurate, as these are obtained from endemic areas where a diagnosis of MTB is not overtly challenging. Similarly, among cases proven by histopathology, the sensitivity of culture has been reported at only 52% [102]. The specificity of MTB culture is 100% [103], as colonization is not known to occur. The pooled sensitivity approaches 100% [104] of PCR-positive cases, but interestingly among cases of clinically diagnosed MTB, the addition of PCR to culture has been reported to increase the sensitivity up to 71% [100] and 74% [99]. IS can be obtained with less invasiveness than bronchoscopy, but the sensitivity reduces to a pooled 58% [103] and 79% [105] of BALF culture-positive cases. Among clinically suspected cases of NTM, a diagnosis that is also easily clinically diagnosed, BALF culture sensitivity has been reported at 84% [94], 93% [104], and 94% [93], and among patients with classical radiographic findings, the sensitivity been reported at only 50% [106]. IS culture sensitivity in clinically diagnosed NTM has been reported at a reduced value of 68% [94], and three consecutive samples are generally recommended to optimize sensitivity. In instances of culture-negative IS during evaluation for NTM, bronchoscopy should be considered. Clinically suspected MTB should be treated with antimicrobial therapy despite negative cultures, as the combined sensitivity of culture and PCR is only modest.
Transbronchial biopsies have been reported to increase the sensitivity of diagnosing MTB an additional 22% [105] compared to that of culture alone, and tissue biopsy should also be considered whenever BALF culture is non-diagnostic.
Serology is infrequently performed, as it does not distinguish active pulmonary tuberculosis from latent infection. ELISA sensitivity for MTB has been reported at 63% to 85% [102] of culture-positive cases, with a specificity of 73% [102] and 100% [102], but lacks commercial availability. Among culture-positive NTM infection, ELISA IgA sensitivity has been reported at 79% [107], with a specificity of 96% [107], but is also not widely commercially available.
BALF interferon gamma release assay (IGRA) ELISA pooled sensitivity has been reported at 90% [108] of clinically diagnosed MTB infection. The low specificity of 80% [108], however, limits its utility with a significant number of false positives. Both serum and BALF IGRA may be positive with either a negative culture or PCR, but both have a significant false-positive incidence that should be judged with a degree of clinical suspicion [109]. The IGRA should be performed on BALF of suspected cases in which culture and PCR are negative, especially if tissue biopsy is deferred, but it is not widely commercially available.
Urine nucleic acid amplification test (NAAT) pooled sensitivity for culture-positive cases has been reported at 55% [110], with a specificity of 94% [110]. Of smear-negative, culture-positive patients, BALF NAAT pooled sensitivity has been reduced to 54% [111], with a specificity of 97% [111]. Due to the low sensitivity of urine NAAT, this method is not recommended.
BALF PCR for MTB pooled sensitivity among culture-positive cases has been reported at 89% [102] and 96% [112], with a specificity of 92% [112] and 99% [102]. The sensitivity of PCR is over 30% [102] higher in those with a positive smear than those without, and for smear-negative cases provides an additional 2% [113] and 15% [100] sensitivity to that of culture alone. Among clinically diagnosed NTM infection, PCR sensitivity has been reported at 67% [93], while the sensitivity in culture-positive NTM infection has been reported at 36% [104] and 87% [95]. PCR for NTM is not now commercially available and requires further evaluation (Figure 2).
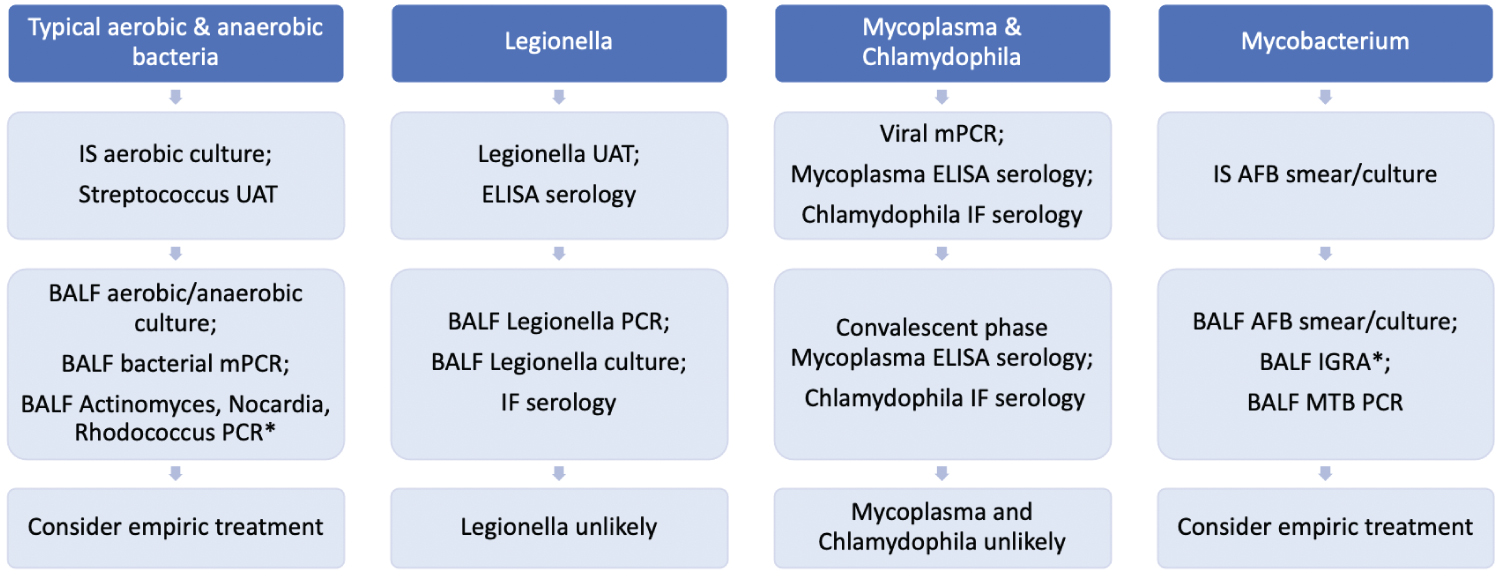 Figure 2: Bacterial pneumonia ("*" Indicates that the test is not widely commercially available). View Figure 2
Figure 2: Bacterial pneumonia ("*" Indicates that the test is not widely commercially available). View Figure 2
Coxiella burnetii is a fastidious intracellular gram-negative bacterium, transmitted to humans through contact with unpasteurized dairy products, goats, sheep, and cows, leading to the disease known as Q fever. There is little evidence on the accuracy of diagnostic testing for Coxiella pneumonia, and the abundance of data relates to endocarditis.
Culture of BALF is discouraged due to risk of transmission to laboratory personnel. The sensitivity of BALF culture is therefore uncertain [114], although in non-respiratory specimens such as heart valves and blood has been reported at 89% [115] of clinically diagnosed cases. Among seropositive patients, the sensitivity of blood culture for acute and chronic infection have been reported at 17% [116] and 53% [116], respectively, but drop close to 0% [116] for patients who are treated with antibiotics at the time of culture.
Various serological methods have been used to detect Coxiella in its two antigenic phases, the initial highly infectious phase I, which is followed by a non-infectious phase II. IF phase II IgM sensitivity has been reported at 89% [117,118] of clinically diagnosed cases and 67% [119] and 91% [117] of cases diagnosed by either seroconversion or a twofold titer increase, with a specificity of 99% [119]. The sensitivity of the IF phase II antibody nearly doubles from the first week of infection to the second week, making serial testing essential [117]. IF Phase I IgG sensitivity for chronic infection is high and approaches 100% [119] of clinically diagnosed cases. ELISA, although not widely commercially available, has a sensitivity reported at 84% [120] and 89% [121] of IF-positive cases, and 95% [117] of those with either seroconversion or twofold titer increase, with a specificity of 99% [120]. Similarly, high-density particle agglutination (HDPA) serology is less sensitive than IF, having been reported at 82% [122] of IF-positive cases, with a specificity of 100% [122]. CF phase I/II IgG sensitivity has been reported at 78% [117] of patients with either seroconversion or a twofold serologic titer increase, but nevertheless is not widely commercially available, is less sensitive than IF, remains elevated for a year, and requires over two weeks to obtain a result [118]. Combining multiple serologic methods increases the sensitivity when compared to that of a single test. For example, the addition of IF and CF to ELISA has been reported to increase sensitivity by 4% [117], while the addition of ELISA and CF to IF has been reported to increase sensitivity by 12% [117]. IF is the only widely commercially available serologic test, however, and is therefore recommended as the initial diagnostic test of choice. Serial testing at two-week intervals should be considered if baseline serology is nondiagnostic.
PCR can be performed on BALF [123] although the accuracy is uncertain. Among serologically diagnosed cases, the sensitivity of tissue PCR of non-respiratory tissue has been reported at 63% [124], 67% [125], 81% [126], and 98% [127], and approaches 100% [126,128] of culture-positive cases, with specificity of 100% [125,127,128]. PCR is commercially available for BALF, and should be considered if serology is negative, however limited the data.
Chlamydia psittaci is an obligate intracellular bacterium comprising eight serovars, commonly acquired through contact with birds.
Culture of BALF or other respiratory secretions is not performed because of its high infectivity and risk to laboratory personnel. Moreover, its sensitivity has only been reported at only 25% [69] of ELISA serology-confirmed cases, 31% [129] of PCR-positive cases, and 63% [130] of clinically diagnosed cases.
Serological methods may be performed, although there is a danger of cross-reactivity with C. pneumoniae. IF IgM sensitivity has been variously reported at 17% [131], 62% [132,133], and 100% [134] of clinically diagnosed cases, and 50% [69] of those positive by either PCR or culture, and can remain elevated for three to four months. CF sensitivity has been reported at only 46% [132] and 59% [133] of clinically diagnosed cases, and 33% [135] of PCR-positive cases. Despite its low sensitivity, CF may be positive in PCR-negative cases and adds up to an additional 40% [135] sensitivity to that of PCR alone. Similarly, IF and CF may be complementary, with CF providing an additional 9% [132] sensitivity to IF with a combined sensitivity of CF and IF reported at 71% [132] of clinically diagnosed cases. ELISA sensitivity has been reported at 75% [136] of clinically diagnosed cases, and 90% [135] of cases positive by either PCR or CF. ELISA and IF serology are the only widely commercially available diagnostic serologic methods, and serial testing should be performed at two-week intervals. Serology is the diagnostic test of choice for Chlamydophila infection but with impaired sensitivity and a lack of alternate laboratory methods, empiric treatment should be considered when there is clinical suspicion.
PCR sensitivity for respiratory secretions has been reported at 35% [75] and 100% [137,138] of culture-positive cases, 44% [139] and 100% [140] of CF-positive cases, 56% [135] of cases positive by either ELISA or CF, 67% [69] of those positive by culture or IF, and 75% [141] of cases positive by IF. PCR is neither suitable for exclusive use nor commercially available.
Francisella tularensis is a facultative intracellular, gram-negative bacterium consisting of a more severe type A subtype and a less severe type B, acquired through contact with hares, ticks, or inhalation of contaminated dust [142]. Ulceroglandular disease is the most common presentation, and data regarding the accuracy for tularemia pneumonia are scarce.
Culture is not generally performed due to the risk of exposure to healthcare workers. Nevertheless, the sensitivity of wound samples has been reported at 78% [143] of PCR-positive cases, and 52% [144] of cases detected by either PCR or serology. The sensitivity of BALF culture for Francisella pneumonia is uncertain.
Serology is the diagnostic test of choice. The sensitivity of ELISA has been reported at 97% [145] of clinically diagnosed cases, 93% [146] and 96% [147] of cases detected by MAG, but reduced to only 63% [144] and 88% [148] of cases detected by either culture, PCR, or four-fold titer increase, with a specificity of 77% [147] and 96% [148]. The sensitivity improves as the time from onset of symptoms lengthens, and approximately doubles between weeks one and three [145]. IgM may remain elevated for months to years after symptom onset [143]. MA sensitivity has been reported at 40% [149] of culture-positive cases, 100% [148] of PCR-positive cases, and 100% [150] of cases proven by EIA seroconversion, with a specificity that approaches 100% [150]. The sensitivity reduces, however, to 75% [148] for cases positive by either culture, PCR, or seroconversion, with a specificity of 99% [148]. IF sensitivity, similarly, has been reported at 73% [148] of cases detected by either culture, PCR, or seroconversion, with a specificity of 99% [148]. Western blotting is an alternative method, but with sensitivity reported at 93% [147] of cases detected by MA, and 100% [150] of cases with EIA seroconversion, with a specificity of 83% [147], is not recommended. Only ELISA serology is widely commercially available and is recommended as the initial diagnostic test, although serial testing should be repeated at a two-to-four-week interval.
PCR sensitivity of non-respiratory tissue has been reported at 93% [151] of clinically suspected cases, 73% [152] and 80% [152] of cases demonstrating seroconversion, and 75% [144], 78% [143], 87% [153], and 90% [144] of cases detected by either culture or seroconversion. The accuracy of PCR on respiratory secretions or BALF is uncertain and requires further evaluation. Regardless, PCR is not commercially available, and if serial serologic titers are negative empiric treatment should be considered.
Yersinia pestis is a gram-negative coccobacillus, transmitted through rodent flea bites or through inhalational exposure to infected respiratory sections.
Cultures derived from sources including lymph node aspirates, urine, sputum and bubo aspirates have a reported sensitivity of 39% [154] of PCR-positive cases, and 45% [155], 53% [154], and 67% [155] of F1 antigen-detected cases. BALF culture may provide additional sensitivity to that of the F1 antigen, but has been poorly evaluated and is not recommended due to the risk to healthcare workers.
Immunochromatographic lateral flow (LFIA) sensitivity for the detection of the F1 antigen has been reported at 59% [154] of clinically diagnosed cases, 73% [154] of PCR-positive cases, and 73% [156] and 100% [154,155,157] of culture-positive cases, with a specificity of 59% [155]. ELISA F1 antigen sensitivity has been reported at 82% [158] and 100% [159] of culture-positive cases, but is not certain to provide additional sensitivity to culture alone. Despite its limited sensitivity and lack of commercial availability, F1 antigen detection is the diagnostic test of choice.
PCR can be detected in both respiratory secretions and tissue aspirates. Tissue PCR sensitivity has been reported in 89% [160] of culture-positive cases, 81% [150] of clinically diagnosed cases, 89% [156] of serologically diagnosed cases by ELISA, and 95% [154] of F1 antigenemia-positive cases. The sensitivity of PCR in sputum has been reported at 70% [161] of culture-positive cases, but is poorly studied in BALF. PCR can be considered when the serum F1 antigen is not detected, but with limited availability of diagnostic testing, empiric treatment should be considered whenever there is clinical suspicion (Figure 3).
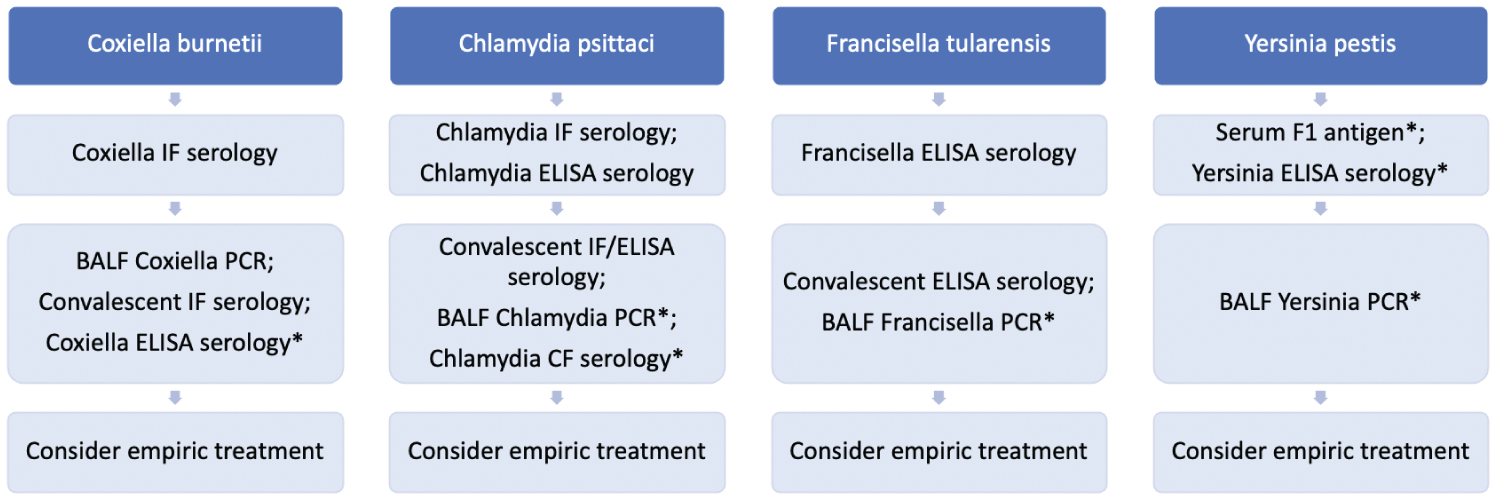 Figure 3: Zoonotic pneumonia ("*" Indicates that the test is not widely commercially available). View Figure 3
Figure 3: Zoonotic pneumonia ("*" Indicates that the test is not widely commercially available). View Figure 3
The accuracy of diagnostic tests vary considerably subject to the reference standard. The sensitivity is falsely low when the reference standard overestimates the true prevalence, such is the case when the standard itself has poor diagnostic accuracy. The corollary is also true, in that sensitivity is falsely high when the referenced standard underestimates the true prevalence of a particular infection. Two diagnostic tests frequently have modest sensitivity when the other serves is a reference standard, illustrating the importance of employing multiple diagnostic modalities. Clinical experience, suggestive history, physical examination findings, and intuition are invaluable. In the absence of diagnostic laboratory investigations, empiric treatment of the clinical diagnosis should be considered.
All authors have contributed equally.
There is no financial support or funding to report.
There are no conflicts of interest to report by any author.