A Percutaneous assist device is commonly used in cardiogenic shock to improve hemodynamics. The Impella provided superior hemodynamic support in the ISAR-SHOCK study compared with an intra-aortic balloon pump (IABP), with no change in clinical outcome. In this trial, the IABP was used in one group, and the Impella was placed in a comparator group. We present a case in which the hemodynamics of cardiogenic shock was assessed in the same patient after IABP and after Impella placement. In our case, the hemodynamics improved after Impella placement and removal of IABP.
Cardiogenic shock (CS) is a common cause of death in acute myocardial infarction. It has been reported that the 30-day mortality rate is up to 60-80% in CS [1,2]. Intra-aortic balloon pump (IABP) or Impella device are the most common devices used in cardiogenic shock to maintain hemodynamics. IABP was downgraded after the IABP-SHOCK II trial failed to show any mortality benefits over medical therapy [3,4]. Impella is an alternative for percutaneous mechanical circulatory support (pMCS), which is widely used as a bridge for recovery. Reports in the literature have shown that the Impella is better than IABP in hemodynamic improvement; however, this improvement was found in different patients and not within the same patients. We report a case in which an Impella was placed after an IABP was removed in the same patient with the goal of improved hemodynamics.
A 68-year-old female with a past medical history of systemic lupus erythematosus, transient ischemic attack, and migraine headaches was admitted for heart failure exacerbation. On admission, a physical exam showed rales bilaterally and +2 lower extremity edema bilaterally. The remainder of the physical examination was unremarkable. Blood pressure was 100/55 mmHg, pulse 110 bpm, and all other vital signs were stable. Electrocardiogram (EKG) showed a slight inferior ST elevation in lead III only with Q-waves, a 1 mm ST-segment depression in leads I and a VL, and an anterior infarct pattern (Figure 1). An old EKG from 2014 also revealed the anterior infarct pattern; however, the ST segment changes were new. Chest X-ray showed diffuse infiltrates bilaterally, and heart size was mildly enlarged (Figure 2). An echocardiogram showed a severely dilated left ventricle and depressed left ventricular systolic function. Left ventricular ejection fraction (LVEF) was 25%, and the total wall motion score was 1.81.
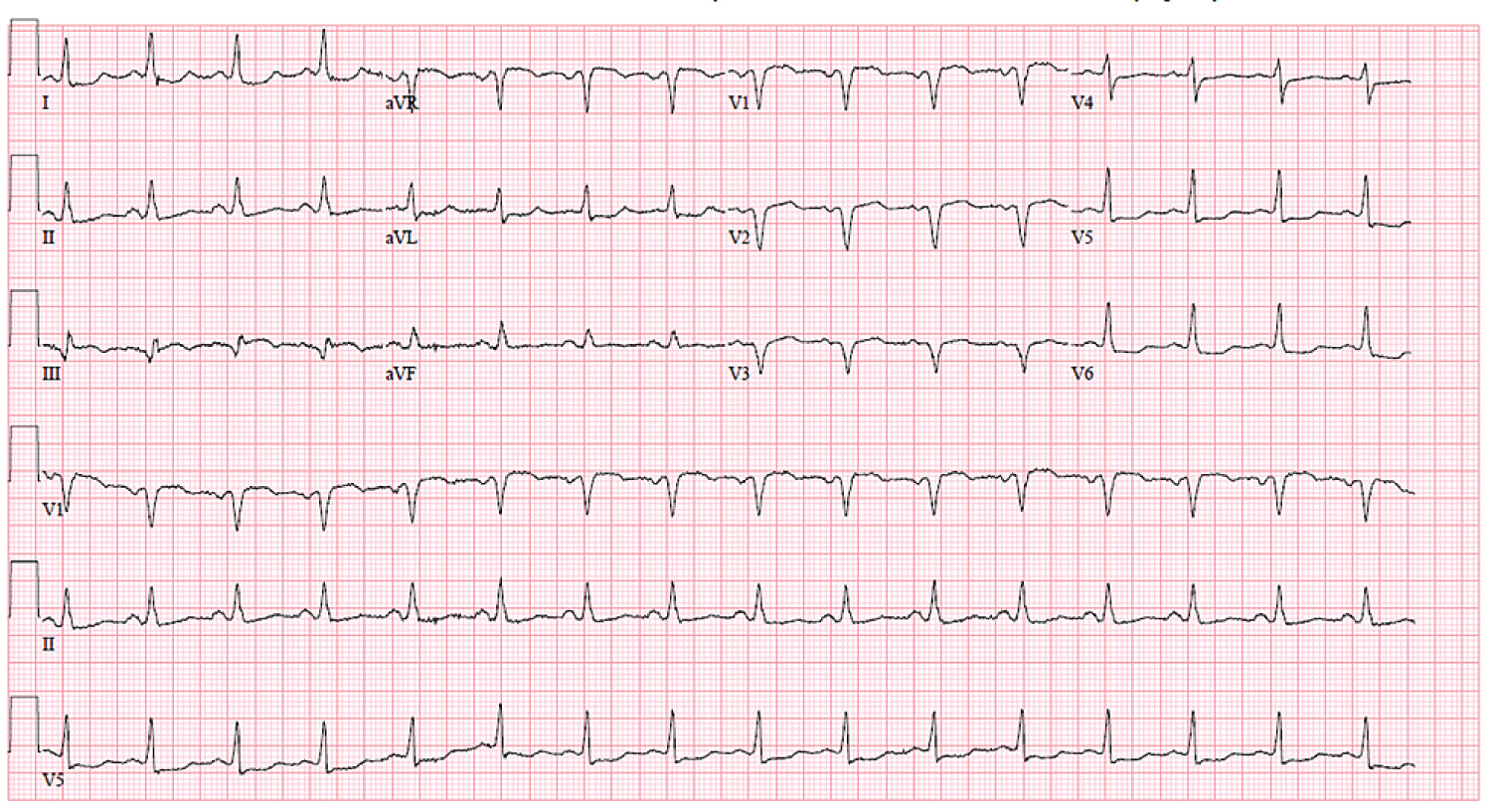 Figure 1: Electrocardiogram showed a slight inferior ST elevation in lead III only with Q-waves, a 1 mm ST-segment depression in leads I and aVL, and an anterior infarct pattern.
View Figure 1
Figure 1: Electrocardiogram showed a slight inferior ST elevation in lead III only with Q-waves, a 1 mm ST-segment depression in leads I and aVL, and an anterior infarct pattern.
View Figure 1
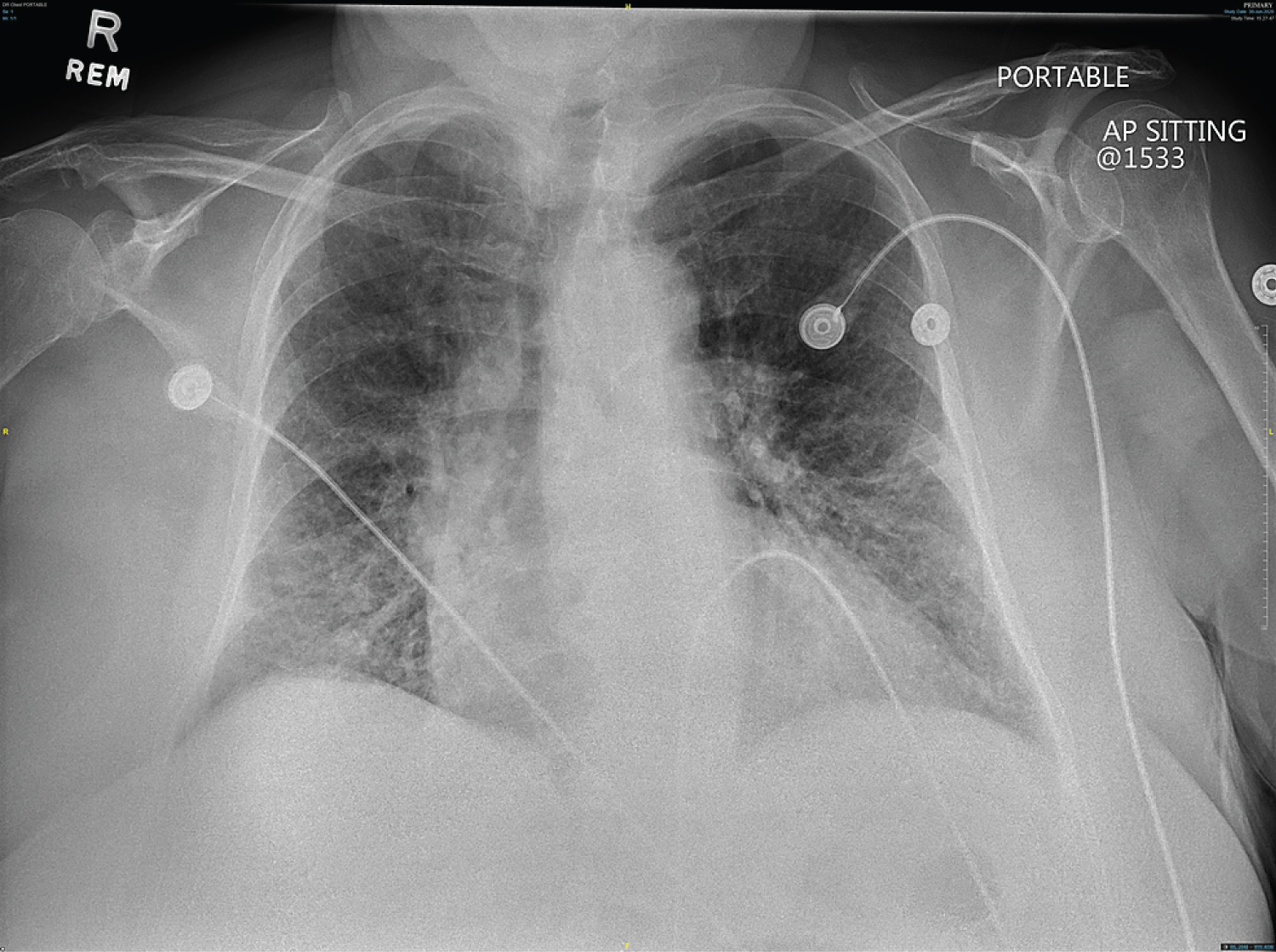 Figure 2: Chest X-ray showed diffuse infiltrates bilaterally.
View Figure 2
Figure 2: Chest X-ray showed diffuse infiltrates bilaterally.
View Figure 2
The Patient's hemodynamics worsened, so an IABP was placed before a heart catheterization. Right and left heart catheterization was performed after IABP placement which showed: A right atrial pressure of 18 mmHg, right ventricular pressure was 50/18 mmHg, pulmonary artery pressure was 52/20 mmHg, a mean of 42 mmHg, and a wedge pressure was 35 mmHg without significant V waves. Total vascular resistance was 1218 dsc-5. The cardiac index was 2.62 l/min/m2, and cardiac output was 5.58 L/min. There was a 90% ostial stenosis, a 50% mid-left anterior descending artery (LAD) lesion with a graftable LAD and diagonal target. After right and left heart catheterization, the Patient started to deteriorate; he developed pulmonary edema and required emergency intubation. A left ventriculogram was performed, which revealed a moderately dilated left ventricular chamber with hypokinesis of the inferior wall, a moderately dilated left ventricular chamber with severe hypokinesis of the inferolateral walls, and mild hypokinesis of the anterior wall. The overall systolic function was impaired with an ejection fraction of 35%. There was a mild 2+ mitral regurgitation found. The ventriculogram did not suggest acute mitral regurgitation due to papillary muscle rupture. At this point, the Impella device was placed, and IABP was removed. The Patient experienced ventricle fibrillation arrest the following day and required shock and cardiopulmonary resuscitation (CPR). The cardiologist adjusted the Impella device under transesophageal echocardiography guidance. Transthoracic echocardiography was performed again the next day, which showed hypokinesis of the entire anterolateral and inferolateral walls with severely depressed ventricular systolic dysfunction left ventricular ejection fraction of 25%. The Patient underwent coronary artery bypass grafting times 3 (left internal mammary artery to LAD, saphenous vein graft to obtuse marginal branch, saphenous vein graft to the posterior descending coronary artery, and endoscopic vein harvest of the left greater saphenous vein). She developed atrial fibrillation (AF) with a rapid ventricular response (AF with RVR) and required four cardioversions at the bedside in a 24-hour period. She was found to be febrile with leukocytosis, which was thought to be due to MSSA associated left basilar pneumonia, and she was treated with cefazolin. She also developed C. difficile colitis and was treated with oral vancomycin. The patient developed torsades at the pointes and was cardioverted. Tracheostomy and percutaneous endoscopic gastrostomy tubes were placed. The patient's condition improved steadily, but she was unable to be weaned off mechanical ventilation. Her condition stabilized, and she was eventually cleared for discharge on ventilator support with tube feeds via percutaneous endoscopic gastrostomy tube. The Patient was admitted again nine months later for gastrointestinal bleed and was later discharged to the rehab facility.
Our case described the benefits of an Impella device for improving hemodynamics in CS when IABP was found to be inferior. Previous studies have shown that Impella improves hemodynamics better than IABP; however, this improvement was noted in different patients. In our case, an IABP was placed first, which did not improve hemodynamics. IABP was subsequently removed, and Impella was then placed, which showed improved hemodynamics.
IABP has minimal effects on preload and cannot independently support the systemic circulation in cases of cardiogenic shock. Impella actively unloads the left ventricle into the ascending aorta and reduces the oxygen demand on the left ventricle. If the IABP does not improve hemodynamics in cardiogenic shock, Impella can maintain cardiac output [5,6]. Studies have also shown that both devices simultaneously showed superior hemodynamics compared to a single device alone [5].
Despite early revascularization, in acute myocardial infarction complicated by cardiogenic shock (AMICS), the mortality is 40-70%. Impella provides superior hemodynamics to explain the significant reduction of lactate and to lower inotropes used in these patients. However, the length of stay, mechanical ventilation rate, and renal replacement therapy (RRT) were not different in patients treated with IABP [7]. Also, the reduction in 30-day mortality with Impella as compared with IABP or standard medical therapy demonstrated a lack of benefits. Some studies suggested a protective effect exerted by Impella in "lower risk" patients. In the "Detroit Cardiogenic Shock Initiative," the early discontinuation of inotropes and vasopressors and the rapid delivery of a pMCS device within 90 minutes of shock development can improve mortality compared to standard medical therapy. Of note, in that study, lower-risk patients were evaluated [7]. In the IABP-SHOCK II study, neither mortality benefit nor harm in patients with CS-AMI randomized to the addition of an IABP to inotropes. In the ISAR-SHOCK study, the Impella LP2.5 provided early, transitory, and slightly superior hemodynamic support compared with an IABP, with no change in outcome. In the ISAR-SHOCK and IMPRESS trials, in severe shock, the pMCS devices were inserted after percutaneous coronary intervention (PCI) for AMI-CS; thus the 30-day mortality reduction was not statistically significant [8,9]. However, a recent study showed that left ventricular unloading before PCI to prevent reperfusion injury could be helpful [10,11]. Therefore, a randomized control trial of Impella 2.5 insertion before reperfusion compared to no MCS is required. The newer generation Impella 5 devices, which are now available, can provide a cardiac output of 5 L/min. Studies have suggested that Impella 5 provides superior outcomes compared to Impella 2.5 in the setting of AMI-CS. Currently, the Impella 5 is limited in daily use as its large profile requires surgical cut-down. Burkhoff, et al. show the native cardiac output increases as the flow of the pump increases by a mechanical circulatory support device. When Impella is placed, the native output is overcome and most of the flow is from the Impella pump. This kind of mechanism was found in the IMPELLA-STIC randomized study [12,13]. In our study, the cardiac output was increased by the impella device. In addition, there is an emerging concept of ECPELLA (venoarterial extracorporeal membrane oxygen [VA-ECMO] and Impella), which would provide a more robust hemodynamic support and is superior to VA-ECMO alone [12]. In our case, the importance of the Impella device implementation was noted in CS as the IABP failed to improve our patient's hemodynamics.
Impella can be substituted for an IABP when further improvement in hemodynamics support is needed in CS.
None declared.
None declared.