On-chip cell sorting is a promising technique for sorting stem cells in culture. On-chip cell sorting allows minimization of lab personnel involvement in cells processing, dramatically reducing the risk of cell culture contamination. We developed a fluorescence-activated On-Chip Cell Culture Sorting (O3CS) system, which combines a biocompatible semiconductor light addressable microarray (chip) and optical setup for chip addressing and cell culture observation. The optical setup has fluorescent and reflected-light microscope capability for visualization and control of cell populations. High-resolution detection of 'unwanted' cells with a high-efficient sorter, based on light-induced electroporation is in the core of the O3CS implemented in NeuroSyntek StemOptimizer 6+. We demonstrated capability of the system to perform cell culture fluorescence activated sorting by inducing irreversible single-cell electroporation, validated O3CS sorting efficacy with fluorescent microscopy and flow cytometry, and compared it with the magnetic-activated cell sorting, demonstrating vastly superior performance in selectivity, efficiency, and sorting speed.
Cell therapy is a fast developing area for the treatment of a wide range of degenerative diseases. High hopes have been associated with the use of Mesenchymal Stem Cells (MSCs). MSCs do not form teratomas, do not cause allogenic rejection versus embryonic stem cells (ES) or induced pluripotent stem cells (iPS). MSCs possess the ability for self-renewing and differentiation into various cell types [1], although they can differentiate into specialized cells to a lesser extent than ES or iPS [2].
Stem cell technologies are widely tested for treating diseases such diabetes [3], heart disease [4], traumatic spinal cord injury [5], Ducheune's muscular dystrophy [6], vision [7], hearing loss [8], and many others. Promising results have been demonstrated in treating cardiovascular diseases [9], neurodegenerative conditions, chronic wounds [10], stroke [11], liver cirrhosis [12], amyotrophic lateral sclerosis (ALS) and acute graft-versus-host diseases GVHD [13]. In ophthalmology, the major emphasis has been aimed at stem cells differentiation into retinal epithelial cells and their progenitors from human embryonic cell lines. These findings, based on existing references [14], have shown safety but limited efficacy.
MSCs obtained from certified cellular banks are claimed to be homogeneous in their stem cell gene expression profile and phenotype. Cell culture procedures, even in GLP-grade laboratories have led to cell transformations and loss or gain of several surface markers [15], indicating that these cells can no longer be used for human treatment. Moreover, prolonged MSCs culturing increases the risk of contamination with different cells, present in the same sample. Mesenchymal stem cells are normally characterized by a set of specific surface markers [16]. MSCs were shown to express CD13, CD29, CD44, CD73, CD90, CD105, CD166, and lack the expression of CD14, CD31, CD45, CD34 usually associated with stem cells committed to hematopoyetic differentiation. In recent years so-called stemness markers among others were discovered. Those stemness markers indicate multipotency of small subpopulation among more differentiated in isolated MSCs [17]. Purification of cells possessing "stemness" markers may justify selection of cells of higher level of multipotency. Nevertheless, it was shown, that expansion of MSCs in vitro according to standard cultivation procedures can lead to modification of surface markers expression levels causing loss of MSC-based therapy efficacy [15].
Samples obtained from human donors, could contain trace amounts of fibroblasts, differentiated or commited stem cells, cancer cells or cancer stem cells, leading to possible negative consequences for their possible use as therapeutic agents. Therefore, when moving from the research bench into clinics it is extremely important to provide high quality separation only the needed stem cells subpopulation from the heterogeneous cells population universe.
Limited number of stem cells sorting methods is available for clinical application. Flow cytomertry is a quite expensive technique; it needs difficult multi-step sample preparation, is time-consuming, cell damaging, bulky, has limited throughput and provides low cell yield after the sorting procedure [18]. Magnetic-activated cell sorting (MACS) is simple in use, provides average-grade cells viability after processing, but leaves concerns related with the magnetic particles themselves and purification quality.
None of the methods existing for stem cells sorting allows operation with stem cells in culture. Before sorting non-hematopoietic stem cells are trypsinized, centrifuged, with all further processing performed at 4 ℃. Multiplicity of preparation steps enhances contamination risks, leading to significant loss in viability, and decreasing the rate of further cells expansion.
On-chip based cell sorting is a fast developing branch of cell sorting techniques. A complete lab-on-a-chip platform would provide the possibility for device miniaturization, simplifying fabrication, reducing costs, continuous cells control, minimizing contamination risks, and integration of various options (cells detection, electroporation, destruction, counting, etc.) in one system. Microchips for cells processing and sorting classified according to the physical principles governing the cell sorting procedure: fluorescence-based, bead-based or label-free sorting [19]. Most of these methods are coupled with microfluidics techniques. Although microfluidic on-chip sorting systems solve problems related to cell sorting (MACS, FACS), they still have to deal with trypsinized cells in suspension and are, therefore, unsuitable for non-hematopoietic stem cells separation.
Electroporation of biological membranes is a recognized and valuable tool in cell biology [20,21]. Pores induced by surges of electrical current through the cell membrane lead to the tremendous increase of ionic permeability. This effect is successfully utilized for transfection of cells, electrofusion as well as permanent cell damage in the case of irreversible electroporation [22].
Standard technique to cause controllable electroporation is the induction of uniform electrical field across of the macroscopic region that contains suspended or adhered cells. The distinctive disadvantage of this technique is non-selectivity to cell characteristics which precludes any manipulation of studied cells. However, electroporating electrical field may be effectively localized by specific microstructures to affect selectively only those cells that comply with specific criteria. Localized electrical field also requires smaller applied potential difference due to non-uniformity of the system.
Different microstructures to obtain specific field localization were proposed, including wired microelectrode arrays, transistor actuated microelectrode arrays and light-addressable microelectrodes. Most of these structures were designed to be used as biosensors to record electrical potential or current. Nevertheless, the application of many of them for the purposes of electroporation is also possible.
Light-addressable microelectrodes and potentiometric sensors are semiconductor devices that leverage light-sensitivity of the substrate [23,24]. They combine very good, adjustable spatial selectivity with simple fabrication procedure. Examples of such devices applied for selective electroporation of biological cells have been presented previously [25-27]. They have been shown to be able to perforate the membrane of a single cell leading to a complete lysis.
In the present study, we propose an accurate, and high-throughput stem cells sorting with a new On-Chip Cell Culture Sorting system - O3CS (designed and built by NeuroSyntek in Silicon Valley). We validated O3CS sorting ability by flow cytometry and fluorescent microscopy, and compared its efficacy with magnetic-activated cell sorting (MACS) in MS columns.
In present study we use a micro-structure of a semiconductor substrate that is capable of achieving far superior characteristics of charge delivery as well as electroporation times. The principal scheme of the developed substrate is illustrated on the Figure 1A.
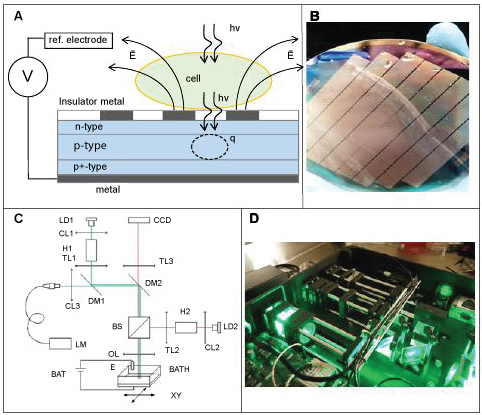 Figure 1: On-Chip Cell Culture Sorting (O3CS) system (NeuroSyntek StemOptimizer 6+).
Figure 1: On-Chip Cell Culture Sorting (O3CS) system (NeuroSyntek StemOptimizer 6+).
A) The structure of O3CS chip and principle of it operation; B) O3CS chip 100 mm wafer; C) Principal optical scheme; D) O3CS optical subsystem.
View Figure 1
Dramatic reduction of time and potential for the electroporation could be achieved by increasing a charge currier transport in the semiconductor as well as by concentrating current at the electrolyte interface with the simultaneous increase of a charge transfer efficiency.
In our design the light-sensitive built in space-charge region extends from 0.4 um to 1 um below the surface. This region shall widen up to the surface under reverse bias potential of several volts, making the system highly photosensitive in the broad wavelength spectrum. At the same time, strong field inside this region swaps photo-generated curriers apart very rapidly, thus effectively suppressing lateral diffusion. As the result, the photocurrent becomes very localized and defined only by illuminated spot. The structure of fabricated chip is schematically illustrated on the Figure 1B.
Light-sensitive substrate was operated by the opto-mechanical block connected to the dedicated microcontroller. Principle optical scheme of this block is presented on the Figure 1C. Optical system was composed of three channels designated for (i) Optical observation, (ii) Epi-luminescence excitation-detection and (iii) Substrate activation. These channels were focused on the light-sensitive substrate, which also formed the bottom of cell culture chamber BATH. Walls of this chamber represented a second electrode E of the electrochemical cell, which was connected to the microcontroller operated voltage source BAT. Cell culture chamber was mounted on the motorized stage XY that permits scanning of optical beams across large areas of the cell culture.
All the components and final assembly of the integrated system for light-induced selective electroporation including substrate, optical setup and microcontroller were designed and fabricated by NeuroSyntek (Los Altos, USA). The system is fully automated, included a removable autoclaved module for continuous stem cells expansion, was coupled with fluorescent and reflected-light microscopy, and was supplied with NeuroSyntek StemOptimizer 6+ software.
In general, O3CS was designed to be a portable 'in culture' cells sorter, providing visualization of stem cells in reflected-light and fluorescent regimes and selective light-induced electroporation and destruction of unwanted cells. The opto-mechanical block is compact - to be placed under the laminar flow in the biosafety cabinet. All operations could be performed in a single sterile environment that significantly reduces risk of culture contamination.
Mesenchymal stem cells were isolated from adipose tissue obtained through a standardized liposuction procedure as described in [28] from healthy patients undergoing cosmetic liposuction. The human breast adenocarcinoma cell line MCF7 and mouse fibroblast cell line NIH/3T3 were obtained from Femsa Biotechnology Center Technolodico de Monterrey (Monterrey, Mexico). Adipose-derived stem cells (ADSCs), MCF7 and NIH/3T3 cells were cultured in DMEM-F12 (Gibco, #12634-010) media supplemented with 10% Fetal Bovine Serum (FBS, Gibco, #10270-106), Penicillin/Streptomycin (Gibco, #15140122) in tissue-treated 10 cm Petri dishes (Corning, #430167) at 37 ℃ in humidified atmosphere with 5% CO2. For NeuroSyntek O3CS testing cells were seeded in two separate wells (30 cm2 each) on the chip with density 5000 cells/cm2.
Cell were magnetically labelled with CD34 MicroBeads (human, Miltenyi Biotec, #130-046-702) according to the protocol provided by supplier, positive and negative sorting was made using MS Columns (Miltenyi Biotec, #130-042-201). Then positively and negatively selected cells were seeded in separate wells on the chip O3CS for further fluorescent microscopy analysis.
Cells were stained with CD 133/2-PE (human, Miltenyi Biotec, #130-090-853) according to the standard FACS staining protocol [29] and cell population heterogeneity was analyzed using BD FACSCanto Flow Cytometer (BD Biosciences).
Cells after MACS or O3CS sorting procedure were seeded on the O3CS chips, stained with CD34-PE, and fluorescent and reflected-light images of cells were obtained. CD34+ cells and total cell number was evaluated through image analysis with Cell^D Soft Imaging System (Olympus Soft Imaging Solution GmbH, Germany).
Sorting efficiency was determined according to equation (1):
where Eexp - experimental efficiency, E100 - maximum efficiency (100% homogeneous cell population, determined as zero percent of cells, stained with CD133/2-PE (or CD34-PE in O3CS vs. MACS experiments), in final population after cells sorting), Ein - initial heterogeneity (% of CD133/2 (or CD34) negative cells in the initial cell population (before separation)), Efin - final heterogeneity (% of CD133/2 (or CD34) negative cells in the final cell population (after separation)).
Cells viability was determined using propidium iodide (PI, Molecular Probes, #P3566) for the experiments with NeuroSyntek StemOptimizer (O3CS) system and 7AAD (Polecular Probes, #A1310) for FACS separation. Staining was performed according to the standard protocols [30].
For the visualization of specific cell surface markers cells were washed twice with Hepes-BSA (140 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 5 mM C6H12O6 with 5% BSA), incubated with 50 μl FcR Blocking Agent (Miltenyi Biotec, #130-059-901) per well on the chip for 5 min at room temperature (RT). Then 50 μl CD105 (or CD133/2, CD34) conjugated to phycoerythrin (PE) was added per each well and cells were incubated for 30 min at RT in dark. Thereafter cells were washed twice with Hepes-BSA. Fluorescence and reflected-light imaging of cells was conducted in Hepes-buffer using 520 nm laser (120 mW) for fluorescence excitation. Fluorescence was registered by CCD camera FL3-GE-50S5M-C (Point Grey Research, Inc. Richmond, Canada) in range 500-750 nm. Reflected-light and fluorescent images in comparison with standard fluorescent microscope (Zeiss) is depicted in Figure 2.
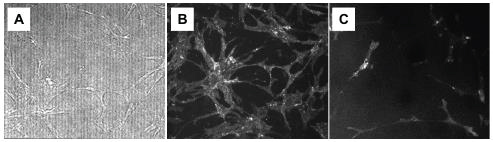 Figure 2: Reflected-light A) And fluorescent B) Images obtained with NeuroSyntek StemOptimizer 6+ (O3CS) in comparison with fluorescent image obtained by Carl Zeiss fluorescent microscope C) ADSCs were stained with CD105-PE. View Figure 2
Figure 2: Reflected-light A) And fluorescent B) Images obtained with NeuroSyntek StemOptimizer 6+ (O3CS) in comparison with fluorescent image obtained by Carl Zeiss fluorescent microscope C) ADSCs were stained with CD105-PE. View Figure 2
NeuroSyntek O3CS is a system which provides a possibility for combination of continuous stem cells culturing with fluorescence-based cell population control (cells labelling with specific surface markers and intracellular staining) and accumulation of the desired stem cell subpopulation through elimination of unwanted cells by means of light-induced electroporation technique [26].
For NeuroSyntek O3CS validation, mixture of CD105-positive and CD105-negative cells (ADSCs and MCF7- respectively) was used. 10% of ADSCs and 90% of MCF7 were mixed together and seeded in two wells of the NeuroSyntek StemOptimizer 6+ chip. In 9 h cells were washed twice with Hepes-buffer solution, incubated with FcR-blocking agent and CD105-PE (50 μl of each in 200 μl of Hepes-buffer solution per each well) for 30 minutes at RT, then washed twice with Hepes. Media was changed for fresh Hepes for automatic light-induced electroporation (LEP).
The O3CS system design provides a possibility to vary the voltage applied to the chip, laser power and pulse duration, thus allowing cells electroporation from mild reversible one to irreversible or even irreversible electroporation with immediate cells swelling and detachment from the chip surface. Blue laser pulse (duration 100 ms) caused destruction of locally selected cell. Propidium Iodide (PI) fluorescence inside the cell enhanced as PI penetrated the cell membrane through LEP-caused pores and intercalated into DNA molecules becoming strongly fluorescent.
NeuroSyntek StemOptimizer 6+ allows for automated separation of cells of interest from the heterogenic population. Data analysis shows, that 100% of CD105+ cells in heterogeneous population were detected by the O3CS system (red crosses on the Figure 3C) and 92% of selected cells were irreversibly electroporated, the process accompanied by significant swelling, destruction of CD105+ cell membranes and cells detaching from the chip surface and floating (Figure 3). The O3CS system is characterized by very high speed of light-induced electroporation of cells, time for the total well chip area (35 cm2) with 10% of cells to be irrevercibly electroporated is less than an hour.
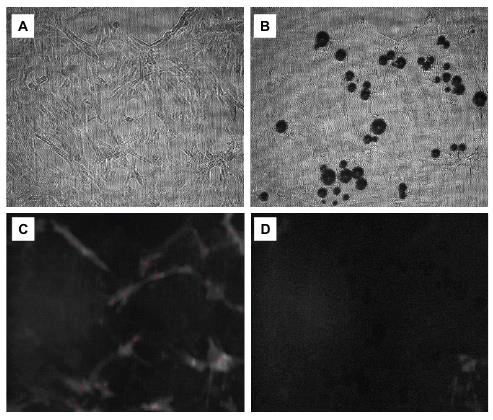 Figure 3: Light-induced electroporation of CD105+ cells. Automated detection, dual imaging (reflected-light and luminescence) of cells and light-induced electroporation, performed with NeuroSyntek StemOptimizer 6+ (O3CS).
Figure 3: Light-induced electroporation of CD105+ cells. Automated detection, dual imaging (reflected-light and luminescence) of cells and light-induced electroporation, performed with NeuroSyntek StemOptimizer 6+ (O3CS).
A,B) Reflected-light images; C,D) Luminescent images. A,C) Cells before LEP, B,D) Cells after LEP. Red crosses indicate cells automatically recognized by O3CS Software as CD105-positive (CD105+).
Cells system under investigation - adipose-derived mesenchymal stem cells (CD105-positive, CD105+) and MCF7 (CD105-negative, CD105-). View Figure 3
NIH/3T3 and ADSCs cells were previously mixed in a tube as shown in Figure 4. Then cells were separated with one portion seeded on the Petri dish for MACS and two portions seeded in the device wells for light-induced electroporation and for a control. Selective electroporation was performed after 18 hours. Cells in one of the device wells were stained with CD34-PE antibody, luminescent and reflected-light images of cells on the chip surface were obtained and light-induced electroporation procedure with NeuroSyntek StemOptimizer 6+ was performed. Negative cells selection, obtained using this technique, was implemented (CD34+-cells were selectively destroyed). Thereafter, cells were additionally stained with PI and second series of fluorescent images of the area exposed to light-induced electroporation were obtained. Cells on the chip were washed with buffer solution to remove fluorescent dyes, fresh DMEM supplemented with 10% of FBS was added and cells were placed into the CO2-incubator and cultivated for 24 h before the next staining iteration. Cells were stained again with CD34-PE in the same area of the chip after 24 hours and fluorescent and reflected-light images were obtained.
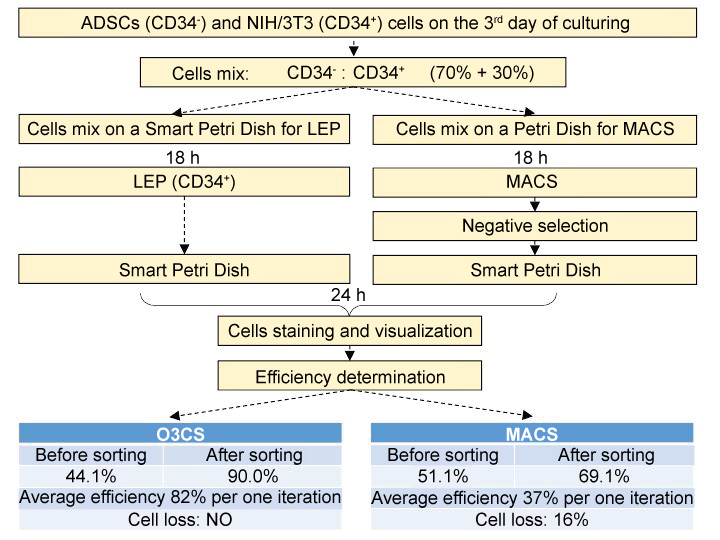 Figure 4: O3CS (Smart Petri Dish) and MACS cells sorting efficiency comparison. ADSCs and NIH/3T3 cells were stained with CD34-PE. CD34+ cells were selectively electroporated by O3CS or excluded with MACS. CD34+ and CD34- cells were counted in a screen-visualized area of O3CS. Percentage of ADSCs in population before and after sorting procedures is provided. View Figure 4
Figure 4: O3CS (Smart Petri Dish) and MACS cells sorting efficiency comparison. ADSCs and NIH/3T3 cells were stained with CD34-PE. CD34+ cells were selectively electroporated by O3CS or excluded with MACS. CD34+ and CD34- cells were counted in a screen-visualized area of O3CS. Percentage of ADSCs in population before and after sorting procedures is provided. View Figure 4
In parallel with O3CS sorting MACS positive and negative separations were performed. Cells from the Petri dish were trypsinized, centrifuged and separated using the MACS. Cells from the positive fraction and cells from the negative fraction were seeded in the wells of the NeuroSyntek StemOptimizer 6+ and placed back into the CO2-incubator. The percentage of CD34+ and CD34- cells was evaluated by means of CD34-PE staining and fluorescent microscopy with NeuroSyntek StemOptimizer 6+ (Figure 4).
These experiments demonstrated that MACS cells separation provides only 37% efficiency per each iteration of separation and at the same time leads to a significant cells loss (> 16% of initial cells number) per each iteration. Cells separation with NS device, in turn, demonstrates 82% efficiency of cells separation with no detectable cells loss per each iteration (Figure 4).
For NeuroSyntek O3CS efficiency estimation a heterogeneous cell system consisted of CD133/2-negative ADSCs and CD133/2-positive MCF-7 was processed with StemOptimizer 6+ system and then tested with flow cytometer, fluorescent microscopy and StemOptimizer 6+ system itself. Flow cytometry showed a 53% efficacy of StemOptimizer 6+system per one iteration (16.1% of CD133/2-positive cells after separation with O3CS left in population over 31.4% of CD 133/2-positive cells in control) (Figure 5).
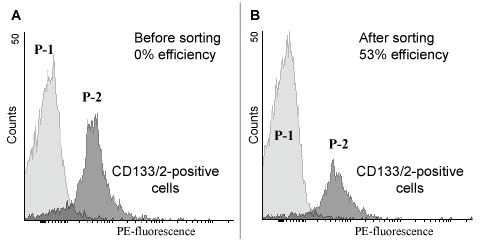 Figure 5: Flow cytometry analysis of cells, sorted with NeuroSyntek StemOptimizer 6+. Histogram plots of expressed CD133/2 surface markers. Heterogeneous cell population consisted of MCF7 cells and ADSCs was stained with anti-human CD133/2 antibodies conjugated to phycoerythrin (PE). CD133/2-positive cells (MCF7) were irreversibly electroporated and removed from cells population through centrifugation. Cells in suspension appeared to form two distinguishable subpopulations as shown by flow cytometry (marked P-1 and P-2 in images).
Figure 5: Flow cytometry analysis of cells, sorted with NeuroSyntek StemOptimizer 6+. Histogram plots of expressed CD133/2 surface markers. Heterogeneous cell population consisted of MCF7 cells and ADSCs was stained with anti-human CD133/2 antibodies conjugated to phycoerythrin (PE). CD133/2-positive cells (MCF7) were irreversibly electroporated and removed from cells population through centrifugation. Cells in suspension appeared to form two distinguishable subpopulations as shown by flow cytometry (marked P-1 and P-2 in images).
A) Distribution of CD133/2-positive cells in control cell population (before LEP); B) Distribution of CD133/2-positive cells in heterogeneous cell population after LEP with NeuroSyntek StemOptimizer 6+. View Figure 5
The efficacy is supposed to be even higher because of the most part of the CD133/2-positive cells detected by flow citometry after StemOptimizer 6+ sorting was estimated to be mortalised (using 7-AAD stain viability test).
There are many techniques for stem cells sorting that include, among others: density gradient centrifugation, pre-plating, dielectrophoresis, field flow fractionation, fluorescence-activated cell sorting (FACS), and magnet-activated cells sorting (MACS). Many of them provide low purity of sorted cells, some can operate only with small amounts of cells and only FACS and MACS are somewhat feasible for their clinical use [31].
However, MACS and FACS require a lot of cell processing and manipulations before the sorting procedure-decreasing cells viability, function, modification of surface marker profiles, and loss in cells quantities. O3CS has several advantages: Minimizing cells manipulations, simultaneous cells expansion, labelling and fluorescent/reflected light imaging with control of cells population. It allows both for single-cell operation and large-scale cells sorting by LEP-technique with high viability of non-operated cells within a single device. O3CS efficacy is better than the ones obtained by FACS, MACS, and fluorescent microscopy.
Overall comparison of O3CS capabilities versus MACS and FACS is shown in Table 1.
Table 1: Comparison of O3CS system with FACS and MACS. View Table 1
The O3CS system is developed to purify heterogeneous stem cell population and expand the cells of interest for further cell therapy applications. It was already used as an effective tool to precisely isolate a specific sub-population of cultured cells suitable for retinal transplantation into human subjects [32]. In the area of ophthalmology the major emphasis is derivation of retinal epithelial cells and their progenitors from human embryonic cell lines. It has demonstrated limited efficacy of identification and separation of RPE cells in the tissue culture using time-consuming manual detection and operation protocols [33]. The O3CS automated approach allows for more cost-effective and higher quality treatment for retina diseases. In addition, O3CS has been tested for safety on animal model (http://media.wix.com/ugd/fac493_8d28a68ee7944d18891791890e6fdabc.pdf).
We thank Dr. David DiGiusto for fruitful discussion and comments.
P.G.M., J.E.M.-C., M.H., C.G., E.W.H., P.M.B., A.A.D., S.N.C. and V.V.B provided ideas formulation, supervised the research activity and reviewed the manuscript, provided expertise. M.T.G.-G., C.L., E.M.Z., R.W., J.M., H.Z., J.K., R.C. and A.Yu.M developed and designed experiment, conducted experiments. T.N.P developed original software and provided software testing and validation. E.N.G conducted experiments and wrote the manuscript. P.G.M., P.M.B. and J.E.M.-C wrote the manuscript. M.T.G.-G. and V.A.S provided study materials, reagents, materials, instrumentation, computing resources. V.V.B secured funding.
The authors declare no competing financial interests.