Introduction: Mivacurium is the shortest-acting non-depolarizing relaxant, used for brief procedures, such as those performed in the ambulatory setting. The recommended intubating dose of 0.2 mg/kg usually provides a clinically effective neuromuscular block for approximately 15 to 20 minutes and spontaneous recovery is 95% complete within about 25 to 30 minutes.
Case presentation: 71 years old woman was admitted for an elective laparotomy surgery with an intraoperative examination. Standard monitoring and TOF Scan (both the adductor pollicis muscle and the orbicularis oculi) were used. 8mg dexamethasone, 0.1mg fentanyl, 150mg propofol, and 18mg mivacurium were administered for induction. At the end of the procedure, there was no response from TOF. The return of neuromuscular transmission was achieved only after 75 minutes from the induction.
Discussion: Mivacurium as a short-acting non-depolarizing muscle relaxant is well suited for short-term operations and operations of unpredictable duration. However, previous studies have shown that an extended neuromuscular block is likely in patients with significantly reduced plasma cholinesterase activity (especially in patients who are homozygous for the atypical plasma cholinesterase gene) as well as when administering some drugs and during other clinical situations.
Conclusions: Extensively prolonged apnoea during general anaesthesia is a dangerous incident. Therefore, we still need systematic reviews to determine the prevalence of incidents of extended neuromuscular block after mivacurium. In case of prolonged muscle relaxation, we should think about possible reversible causes of this phenomenon since it is not always related to genetic causes. Furthermore, mechanical ventilation and close clinical monitoring are required during administering mivacurium. All of this to achieve the best possible patient outcomes.
Mivacurium, Prolonged duration of action, Plasma cholinesterase, Neuromuscular block, TOF, Non-depolarizing
Mivacurium is a mixture of three stereoisomers that competitively block cholinergic receptors within the motor plate, resulting in skeletal muscle relaxation. It is a potent non-depolarising neuromuscular blocking agent that has a short-acting duration because of its rapid elimination by plasma cholinesterase. Therefore, it's the drug of choice for short procedures where we want relaxation to resolve before the end of the procedure (e.g. for laryngeal microsurgery or thyroidectomy with intraoperative neuromonitoring). The recommended intubating dose (2 × ED95) usually provides a clinically effective neuromuscular block for approximately 15 to 20 minutes and spontaneous recovery is 95% complete within about 25 to 30 minutes [1,2]. Consequently, in case of long procedures, where mivacurium was used for intubation, and long-term relaxation is needed, the appropriate approach would be to extend it with a non-depolarizing drug, most often rocuronium. However, previous studies have shown that an extended neuromuscular block is likely in patients with significantly reduced plasma cholinesterase activity (especially in patients who are homozygous for the atypical plasma cholinesterase gene) [3]. The second observed adverse effect was linked to mivacurium-induced histamine release, causing cardiovascular effects such as hypotension and cutaneous flushing. The following clinical case report provides information on what to be aware of when administering the relaxant mivacurium.
On 19/05/2021 a 71-year-old woman was admitted to the Gynecology Department of The Regional Specialist Hospital in Olsztyn, Poland. She was referred to the hospital for an elective laparotomy due to the diagnosis of a tumor of the right appendages and endometrial hyperplasia. This patient had obesity (height = 165 cm, weight = 93kg, BMI = 34.16kg/m2), suffered from persistent atrial fibrillation and had a history of cholecystolithiasis. She received a score of 2 in the ASA classification. She had no history of genetically determined abnormalities of plasma cholinesterase or muscle disorder. On a daily basis, she only takes Neoparin (Enoxaparin sodium) 40mg 1x1 and Concor (Bisoprolol) 1,25mg 1x1. She was rated 1 on the Mallampati scale. She didn't receive any premedication.
The next day the patient arrived at the operating room. The patient was also in a clinical trial comparing the TOF Scans, therefore we measured the neuromuscular transmission with this device through the electrodes placed above the ulnar nerve (the adductor pollicis muscle-TOFapb + standard method) and on the forehead (the corrugator supercilii muscle - TOFcsm). After securing the perioperative monitoring: EKG, NIBP, SaO2, ventilation parameters, an epidural blockade L3-L4 was performed with 10ml Bupivacaine Hydrochloricum 0.5%, 6ml Fentanyl (0.1mg/2ml) and 34ml NaCl at 6 milliliters per hour. Dexamethasone 8mg as an antiemetic, 0.1 mg fentanyl, 150mg propofol, and 18mg mivacurium were used for induction. The patient was intubated after 135 seconds with a 7.0 tube using a bougie guide (Cormack grade 2). Anaesthesia was maintained with a mixture of sevoflurane (MAC 0.9) and air with FiO2= 0.5, FGF = 1 l / min. Ventilation was carried out in SIMV-PC mode with PEEP = 5mbar, without pressure support. An intravenous infusion of 1000ml of Optilyte was administered. The neuromuscular transmission was measured every 15 seconds during the first 10 minutes and then every 5 minutes till extubation. After 30 minutes, TOFapb-Scan still showed 0%. TOFapb > 90% was obtained only after 75 minutes after the administration of a single dose of mivacurium (Figure 1). The patient was extubated without any complications after 80 minutes with TOFapb = 96% and received 10 points in Aldrete score.
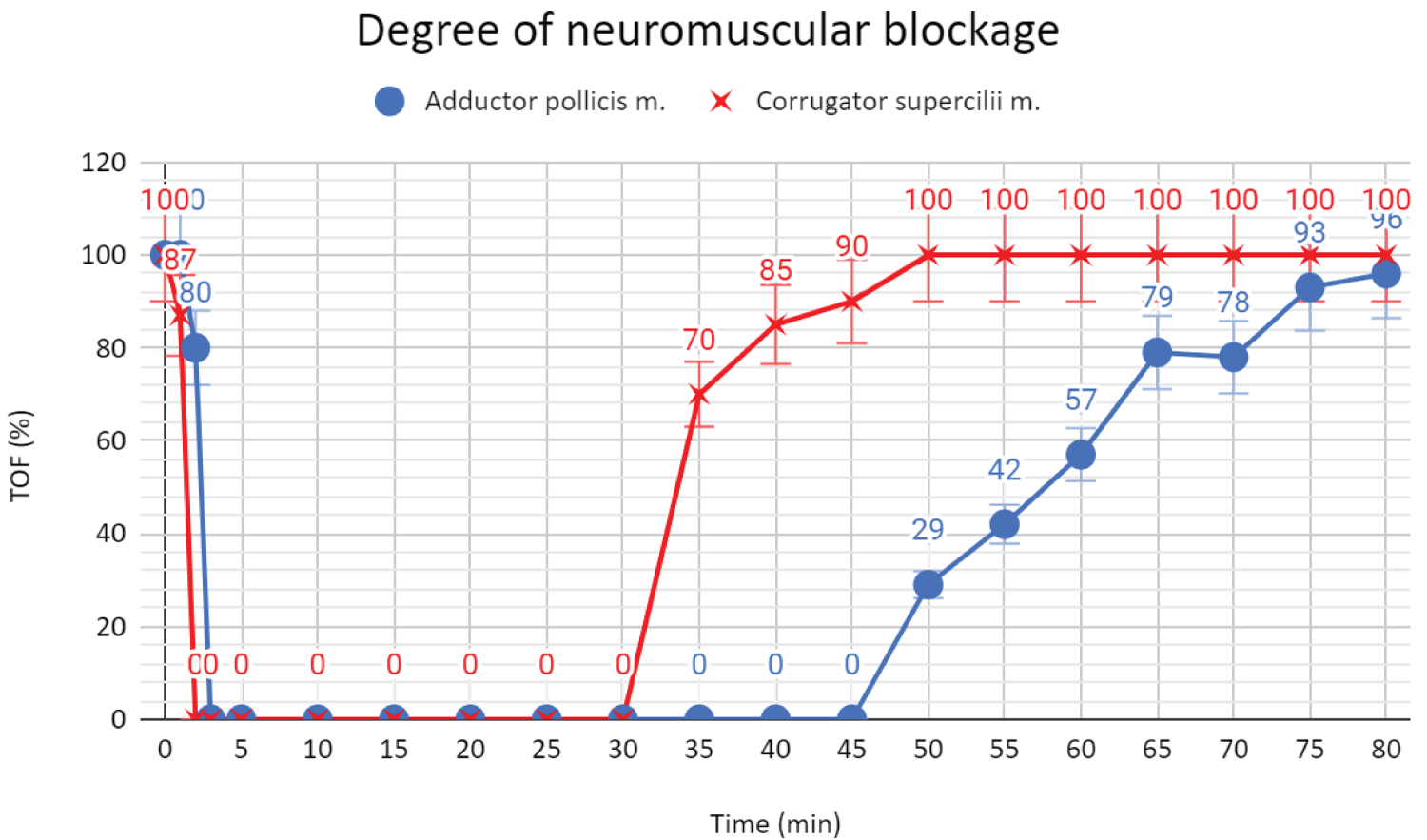 Figure 1: Degree of neuromuscular blockage.
View Figure 1
Figure 1: Degree of neuromuscular blockage.
View Figure 1
Mivacurium belongs to the group of non-depolarizing, highly specific, short-acting muscle relaxants. However, we have to keep in mind that significantly reduced plasma cholinesterase activity (especially in patients who are homozygous for the atypical plasma cholinesterase gene) and mutations in the butyrylcholinesterase enzyme (BChE) may prolong the effect of the neuromuscular blocking agents such as mivacurium and succinylcholine. Heterozygotes can have a muscular paralysis of moderate duration, while homozygotes can experience muscular block longer than 2 hours [4]. This is due to the fact that succinylcholine is also degraded by plasma pseudocholinesterase (pChe). Previous studies have shown several mutations in the BChE (I373T, G467S, W518R, L184S, V421A, M462I, and R577H) that can cause extensively prolonged apnoea during general anaesthesia when mivacurium or succinylcholine are used [5]. Injection of human cholinesterase increases pChe activity and may help to shorten the duration of action of mivacurium three to four times. Another medicine that can be of some use is neostigmine. When administered after cholinesterase it increases the rate of recovery further, albeit full recovery may be slow in some patients [6,7]. When diagnosing the causes of prolonged neuromuscular block, it should also be remembered that the intensification of the mivacurium effect may be caused by: inhalation anaesthetics (apart from nitrous oxide), antibiotics (including aminoglycoside antibiotics, polymyxins, tetracyclines, lincomycin, clindamycin), antiarrhythmic drugs (propranolol, calcium antagonists, lidocaine, procainamide, quinidine), diuretics (furosemide, possibly thiazide diuretics, mannitol, acetazolamide), magnesium salts, lithium salts, ketamine, ganglion blockers. Drugs that reduce the effect of plasma cholinesterase may increase the effect of mivacurium - these include antimitotic drugs, pancuronium, organophosphorus compounds, some hormones, MAO inhibitors, and plasma cholinesterase inhibitors. Some drugs may worsen or induce latent myasthenia gravis, increasing the sensitivity to the effects of mivacurium, including various types of antibiotics, γ-blockers, and anti-arrhythmic drugs (procainamide, quinidine), chlorpromazine, anti-rheumatic drugs (chloroquine, D-penicillamine), steroids, phenytoin, lithium salts. Hypothermia may also prolong the duration of action [8]. Additionally, the clinically effective duration of block may be about 1.5 times longer in patients with end-stage kidney disease and about 3 times longer in patients with end-stage liver disease than in patients with normal renal and hepatic function. Severe acid-base and/or electrolyte abnormalities may also potentiate or cause resistance to the neuromuscular blocking action of mivacurium [9]. However, none of the causes mentioned above was the reason for prolonged neuromuscular blockade in our patient since she had no hypothermia, electrolyte abnormalities, renal or hepatic disease, etc. It is also important that even if the TOF ratio is 0%, it is possible to observe the patient's respiratory movements because the diaphragm and respiratory muscles are the least susceptible to relaxation of the skeletal muscles. [10]. This means that the indications of the ventilator, or even the patient's respiratory movements, are not a good prognostic factor for the receding relaxation.
Extensively prolonged awakening during general anaesthesia is a dangerous incident. The drug should be administered in individually selected doses under the supervision of an experienced physician who knows the effects of the drug and possible complications associated with its use. We should not administer mivacurium if the conditions for resuscitation, oxygen therapy, and mechanical ventilation are not provided, or if drug antagonists are not within immediate reach. Mechanical ventilation and close clinical monitoring are required during administering this drug. In case of prolonged muscle relaxation, we should think about possible reversible causes of this phenomenon since it is not always related to genetic causes. Furthermore, we still need systematic reviews to determine the prevalence of incidents of extended neuromuscular block after mivacurium. All of this to achieve the best possible patient outcomes.
None.
None.
Data collection: Paweł Radkowski, Katarzyna Podhorodecka, Mariusz Keska
Data analysis: Paweł Radkowski, Katarzyna Podhorodecka, Mariusz Keska
Writing of paper: Paweł Radkowski, Katarzyna Podhorodecka, Mariusz Keska
Revising of paper: Paweł Radkowski, Katarzyna Podhorodecka, Mariusz Keska