Background and aims: To determine actual concentration of clinically significant IgG antibodies, there is need to inactivate IgM antibodies. Aim of this study was to study effect of dithiothreitol (DTT) on ABO isoagglutinin titers performed by column agglutination technology (CAT) and conventional tube technique (CTT) and to compare results obtained by DTT treatment.
Materials and methods: This was a prospective, observational study conducted from October 2018 to March 2020. All consecutive O group donors were included. All samples were consecutively tested by CTT and CAT, before and after DTT treatment (pCTT, pCAT).
Results: A total of 2005 donors were included. 295 (14.71%) had inter-observer variation by CTT and 51 (2.54%) had inter-observer variation by CAT. IgG titers were found to be more than IgM titers. Reduction in IgG titers was observed with DTT. Concordance between CTT and pCAT was found to be unsatisfactory. pCTT results showed one-fold higher decrease than pCAT when compared to pre-treatment results. IgG titers for both anti-A and anti-B showed strong correlation between CTT (1+ strength) and pCAT (2+ strength). Comparison between CTT (1+) and CAT (1+, 2+, 3+), pCTT (1+) and CAT (1+, 2+, 3+), pCTT (1+) and pCAT (1+,2+, 3+) was performed using Wilcoxon signed-rank paired test for significance. For all comparisons, test was significant at 1+ and not significant at 3+ end point.
Conclusion: There is significant difference between antibody titers estimated using DTT treated and untreated plasma. Use of DTT for ABO isoagglutinin titer estimation is recommended.
Isohemagglutinin titer, ABO, Conventional tube technique, Column agglutination technique, DTT
Determination and monitoring of isoagglutinin titres play an important role in the outcome of solid organ and hematopoietic stem cell transplant. IgM and IgG antibodies are the two antibodies which play an important role in transfusion science. [1,2]. Anti-A and anti-B antibodies belonging to individuals of A and B blood group are predominantly IgM type while those of blood group O are predominantly IgG type. While testing for IgG antibody titers, their concentration can be masked by IgM antibodies [3]. In order to determine the actual concentration of the clinically significant IgG antibodies, there is a need to inactivate IgM antibodies. Literature provides methods to inactivate IgM antibodies such as heat inactivation at 63 °C or use of sulfhydryl reagents such as 2-mercaptoethanol (ME) and dithiothreitol (DTT; also called Cleland's reagent) [4,5]. DTT is a sulfhydryl compound which inactivates IgM antibodies by cleaving inter subunit disulphide bonds [6,7]. Treatment with DTT has been proven to be superior to treatment with 2-ME in terms of no obnoxious odour and no need for dialysis of the specimen in some cases [6,8,9]. IgG antibodies are less susceptible to DTT because the disulphide bonds between the chains are not as labile as those of IgM subunits [10,11]. But they may get slightly affected [12]. As DTT inactivates IgM antibodies, routine use in clinical laboratories has been recommended where IgM interference is suspected [6,13].
Conventional tube technique (CTT) has been widely used as a method of antibody titration. However, CTT being a manual method, has certain disadvantages; it is time consuming, labor-intensive, has inter-observer as well as inter-laboratory variations. Like other immunohematological investigations, titration is being offered on various automated immunohematology analyzers with the advantage of high throughput, less inter-observer and inter-laboratory variation and easy for laboratory personnel. These analyzers are based on different techniques like column agglutination technology (CAT) and solid phase red cell adherence (SPRCA)/hemagglutination (HA). There are studies which compare results obtained using different methods of titration [14-19]. Many of these techniques conclude that the results of the age old CTT do not correlate with the results obtained by newer techniques [20-24].
The aim of the present study was:
1. To study the effect of DTT on ABO isoagglutinin titers performed by CAT and CTT
2. To compare results of ABO isoagglutinin titers obtained by DTT treatment performed by CAT and CTT
This was a prospective, observational study conducted in the department of Transfusion Medicine at a tertiary healthcare center from October 2018 to March 2020. All consecutive O blood group donors were included samples were simultaneously tested by CTT and CAT for anti-A and anti-B titration. For studying the effect of DTT, serum from each donor was treated with DTT and tests were performed by CTT and CAT. All results were recorded for comparison.
All consecutive O blood group donors who were eligible to donate blood as per the guidelines laid down by the Drugs and Cosmetics Act, 1940 and the Standards for Blood Banks and Blood Transfusion Services were included in the study [25,26]. Pilot tubes collected at the time of donation were used for titration. No additional samples were drawn. After performing the routine donor testing, antibody titration was performed from the residual sample either on same day or on the next day of collection. If tested on the next day, the sample was stored at 4 °C. All donors who did not give consent to participate in the study, donors reactive for transfusion transmitted infections, samples with positive direct antiglobulin test or positive antibody screen were excluded from the study.
Conventional test tube technique (CTT): Titration was performed by CTT according to the method described in AABB technical manual [3]. The titer end point was the reciprocal of the lowest dilution yielding 1+ agglutination with naked eye. The reactions were recorded for IgM and IgG on a case reporting form.
Column agglutinnation technique (CAT): For IgM titer, Neutral Ortho BioVue System cassettes (Ortho Clinical Diagnostics, Raritan, New Jersey, USA) were used while for IgG, Anti-IgG Monospecific Ortho BioVue System cassettes (Ortho Clinical Diagnostics, Raritan, New Jersey, USA) were used. Dilutions of test sample were prepared as for CTT and testing was performed as per manufacturer's instructions. The reactions were then read and recorded on a case reporting form. The titer end point was the lowest dilution yielding 1+, 2+ and 3+ agglutination visible to the naked eye.
DTT preparation: 0.01M DTT was prepared by dissolving 0.154 g of DTT in 100 ml of PBS (pH 7.3) as described in the steps detailed in AABB technical manual [3].
DTT treatment of serum: Serum was treated with 0.01M DTT as per the method described in AABB technical manual [3]. One volume of the prepared DTT was combined with one volume of serum. The mixture was incubated at 37 °C for 30 to 45 minutes mixing every 5 minutes. From this mixture serial dilutions were prepared and antibody titration was performed using column agglutination technique for both IgM and IgG. For dilution control, one volume of patient serum was mixed with one volume of PBS and that mixture was used for serial dilutions and titration. This was done to show that reactivity was not reduced simply by dilution of serum.
To reduce inter-observer bias for manual (CTT) and semi-automated (CAT) methods, each sample was given to two different personnel to perform the test. Results obtained were given to the Transfusion Medicine physician. Final results were declared by the Transfusion Medicine physician.
Data was entered in an MS excel sheet; numerical values, percentages, mean and standard deviation was calculated. Statistical analysis was performed using SPSS software (Version 25.0.0.0, Chicago, USA). Median IgM and IgG titres were calculated for anti-A and anti-B obtained by CTT and CAT at three different end points (1+, 2+ and 3+); both pre and post DTT treatment. Concordance between CTT results (1+ strength) and results obtained by CAT (before and after DTT treatment) with 1+, 2+, and 3+ strength of reaction as end point was calculated. Correlation between the methods was tested using Spearman's rho for the first 200 samples of blood group O and all samples of blood group A and B. The strength of the correlation was calculated using the following guide for the absolute value of rs:
0.0-0.19 - very weak
0.20-0.39 - weak
0.40-0.59 - moderate
0.60-0.79 - strong
0.80-1.0 - very strong
Nonparametric Wilcoxon signed-rank paired test was used to test for significance comparing IgG results between CTT and CAT, pCTT and CAT and pCTT and pCAT used for a given sample. For this purpose, a total of 10 samples (every 200th sample) was included.
All donors who gave informed consent to participate in the study were included. For the purpose of the study, all data was identified with donor unit numbers only. No extra blood sample was collected; tests were performed using pilot blood samples meant for blood grouping and testing for transfusion transmitted infections. The study was approved by the institutional review board (IRB) and the institutional ethics committee (IEC).
A total of 2005 O blood group, healthy, whole blood donors participated in this study out of which 1916 (95.5%) were male and 89 (4.4%) were female. Mean age of the participants was 32.2 ± 8.05 years. A total of 295 (14.71%) samples had inter-observer variation by CTT and 51 (2.54%) samples had inter-observer variation by CAT. Figure 1 illustrates the distribution of anti-A and anti-B titers, IgG and IgM titers performed by CAT before and after DTT treatment with 1+ strength as end point. For both Anti-A and Anti-B, IgG titers were found to be more than IgM titers. A reduction in both IgG and IgM titers was observed with DTT treatment. While distribution of anti-A and anti-B IgG titer results were found to be similar before DTT treatment, Anti-B IgG titer results after DTT treatment were found to be higher than post treatment Anti-A IgG titer results.
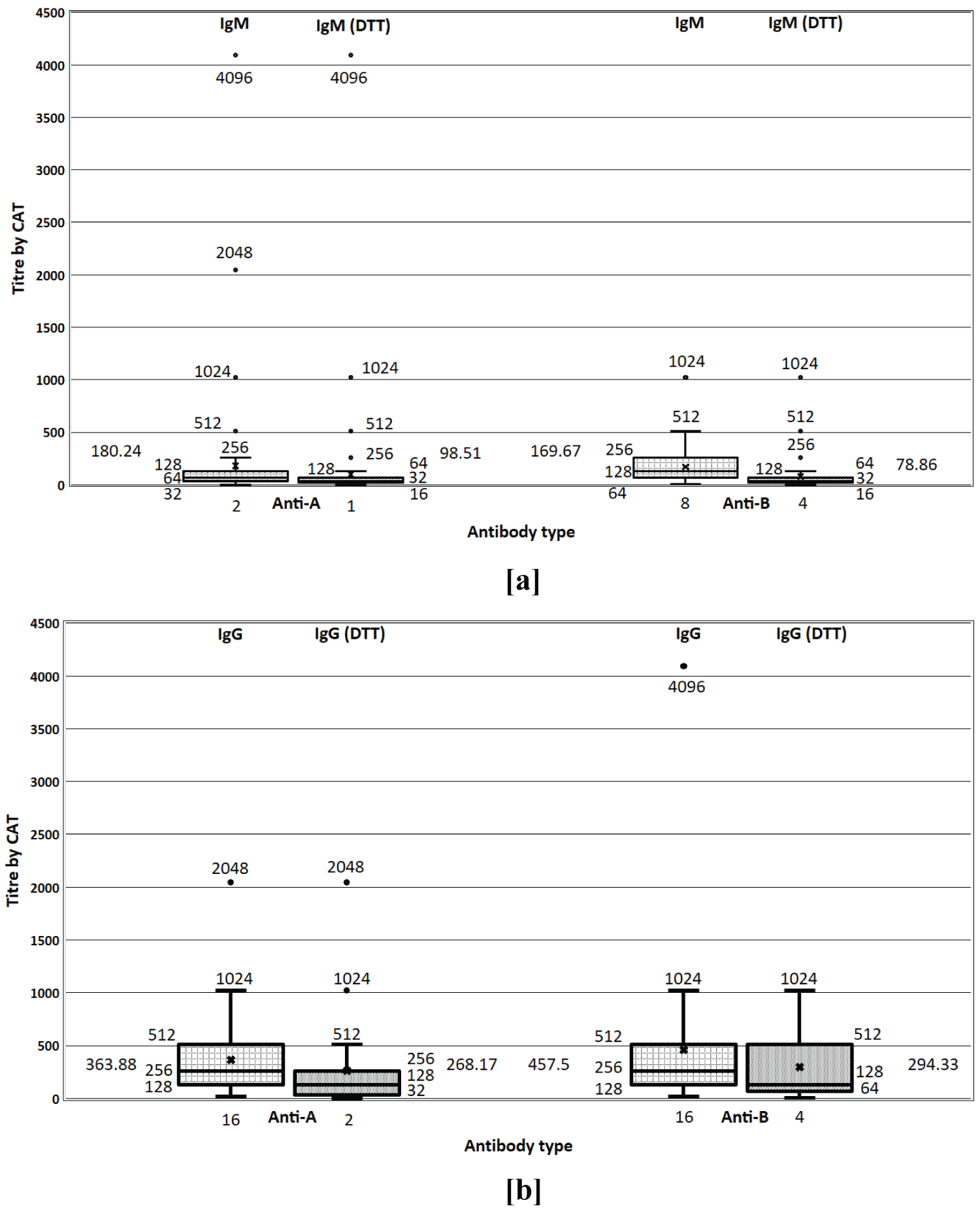 Figure 1: Distribution of anti-A and anti-B titers performed by CAT. a) IgM titer before and after DTT treatment; b) IgG titer before and after DTT treatment.
View Figure 1
Figure 1: Distribution of anti-A and anti-B titers performed by CAT. a) IgM titer before and after DTT treatment; b) IgG titer before and after DTT treatment.
View Figure 1
Concordance between CTT results at 1+ strength as end point and pre DTT treatment CAT at 1+, 2+ and 3+ strength end points was calculated and has been listed in Table 1 for IgM titers and in Table 2 for IgG titers. There was 43.4% and 40.3% agreement of the anti-A IgM and IgG titers between CAT (2+ strength) and CTT (1+ strength) respectively. There was 43.6% and 32% agreement of the anti-B IgM and IgG titers between CAT (2+ strength) and CTT (1+ strength) respectively.
Table 1: Concordance of IgM titer results obtained by CTT (1+ strength as end point) with pre-DTT and post DTT treatment results obtained by CAT (1+/2+/3+ strength as end point). View Table 1
Table 2: Concordance of IgG titer results obtained by CTT (1+ strength as end point) with pre-DTT and post DTT treatment results obtained by CAT (1+/2+/3+ strength as end point). View Table 2
Concordance between CTT results at 1+ strength as end point and post DTT treatment CAT (pCAT) at 1+, 2+ and 3+ strength end points was calculated and has been listed in Table 1 for IgM titers and in Table 2 for IgG titers. There was 3.5% and 11.5% agreement of the anti-A IgM and IgG titers between pCAT (3+ strength) and CTT respectively. There was 0.8% and 10.9% agreement of the anti-B IgM and IgG titers between pCAT (3+ strength) and CTT respectively. There was 15.4% and 21.3% agreement of the anti-A IgM and IgG titers between pCAT (2+ strength) and CTT respectively. There was 14.8% and 30.9% agreement of the anti-B IgM and IgG titers between pCAT (2+ strength) and CTT respectively. There was 24.2% and 28.7% agreement of the anti-A IgM and IgG titers between pCAT (1+ strength) and CTT respectively. There was 30.2% and 17.5% agreement of the anti-B IgM and IgG titers between pCAT (1+ strength) and CTT respectively. The concordance between CTT and pCAT was found to be unsatisfactory across all categories.
Figure 2 shows the comparison between median IgG and IgM titers for anti-A and anti-B by CTT and CAT, both pre DTT and post DTT (pCAT and pCTT) treatment with results interpreted at 1+, 2+ and 3+ end points. Median IgM titers for anti-A and anti-B were found to be lower than median IgG titers. The median titer of anti-A IgG were highest in CAT (1+ strength- 256) which was followed by CTT (1+ strength- 64). The median titers of anti-A IgM were same for CAT and CTT (1+ strength- 64). The median titer of anti-B IgG was highest in CAT (1+ strength- 256), followed by CTT. The median titer of anti-B IgM was highest in CAT (1+ strength- 128), followed by CTT (1+ strength- 64). Median titer obtained by pCTT across all categories were lowest whereas those obtained by CAT were highest. For IgG titers, median anti-A and anti-B titers obtained by CTT (1+) and pCAT (2+) were identical and median anti-A and anti-B titers obtained by pCTT (2+) and CTT (3+) were identical. For IgM titers, median anti-A and anti-B titers obtained by pCTT (1+) and CTT (3+) were identical. When considering CTT IgG median titers, one to two-fold decreases was observed with DTT treatment. However, for CAT IgG median titers, only one-fold decrease was observed with DTT treatment. In contrast, when considering CTT IgM titers, two to three-fold decrease was observed with DTT treatment. However, for CAT IgM median titers, only one to two-fold decreases was observed with DTT treatment.
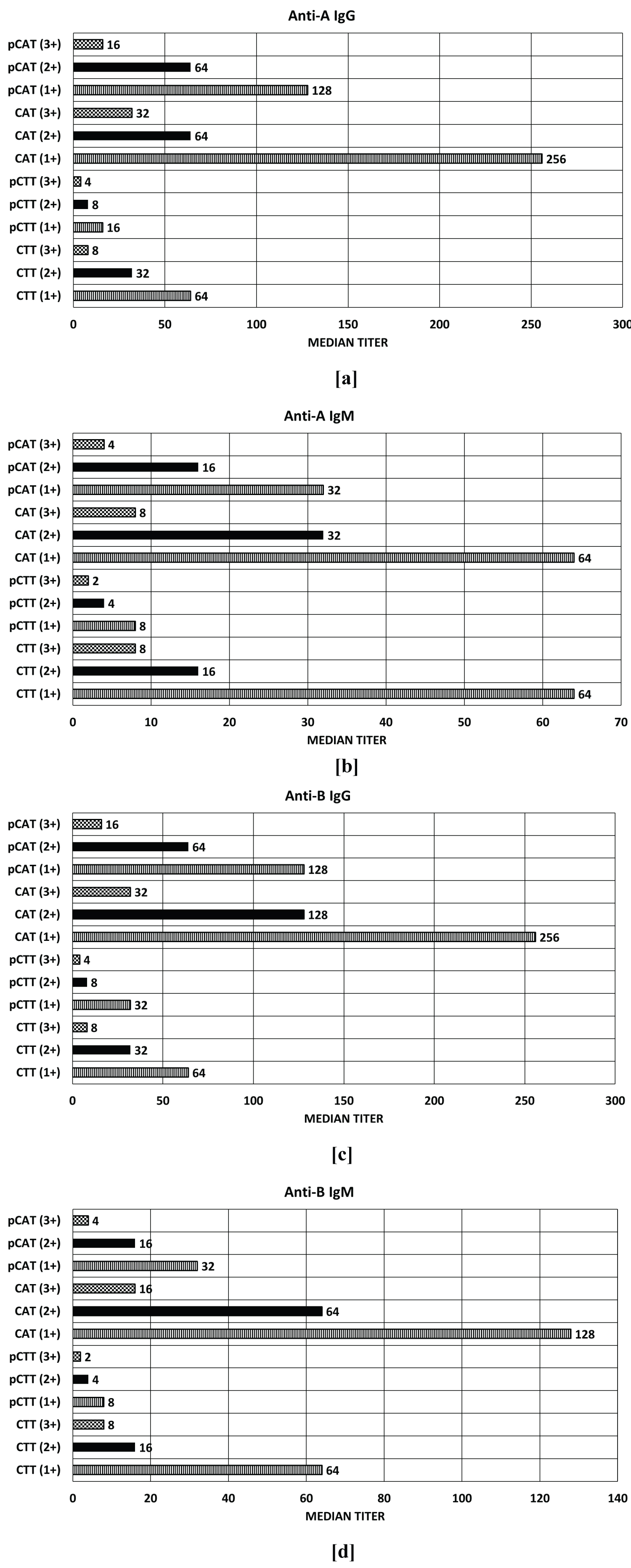 Figure 2: Comparison of median anti-A and anti-B titers before and after DTT treatment performed by CTT and CAT at 1+/2+/3+ end points. a) Median anti-A IgG titers; b) Median anti-A IgM titers; c) Median anti-B IgG titers; d) Median anti-B IgM titers (CAT, CTT- Pre DTT treatment; pCAT, pCTT - Post DTT treatment).
View Figure 1
Figure 2: Comparison of median anti-A and anti-B titers before and after DTT treatment performed by CTT and CAT at 1+/2+/3+ end points. a) Median anti-A IgG titers; b) Median anti-A IgM titers; c) Median anti-B IgG titers; d) Median anti-B IgM titers (CAT, CTT- Pre DTT treatment; pCAT, pCTT - Post DTT treatment).
View Figure 1
Table 3 lists the Spearman's rho (rs) for correlation between CTT (1+ strength) and CAT (1+, 2+ and 3+ strengths) and between CTT (1+ strength) and pCAT (1+, 2+ and 3+ strengths) for the first 200 samples. The statistical analysis was performed for IgM and IgG titers for both anti-A and anti-B antibodies individually. The results show that IgM and IgG measurement of anti-A and anti-B showed strong correlation between CTT (1+ strength) and CAT (1+, 2+ strengths). Similarly, IgM and IgG titers for both anti-A and anti-B showed strong correlation between CTT (1+ strength) and pCAT (2+ strength).
Table 3: The correlation of pre DTT treatment and post DTT treatment results obtained by CTT (1+) and CAT (1+,2+,3+) measuring IgG and IgM antibodies for anti-A and anti-B. View Table 3
Figure 3 illustrates the IgG results obtained for every 200th sample. A comparison between CTT (1+) and CAT (1+, 2+, 3+), pCTT (1+) and CAT (1+, 2+, 3+), pCTT (1+) and pCAT (1+, 2+, 3+) was performed using Wilcoxon signed-rank paired test for significance. It was observed that for all the above mentioned comparisons, the test was significant at 1+ end point and not significant at 3+ end point. For CTT (1+) and pCAT (2+), the test was not significant for anti-A and anti-B. For pCTT (1+) and pCAT (2+), the test was significant for anti-A and anti-B. For CTT (1+) and CAT (2+), the test was significant for anti-B and not significant for anti-A.
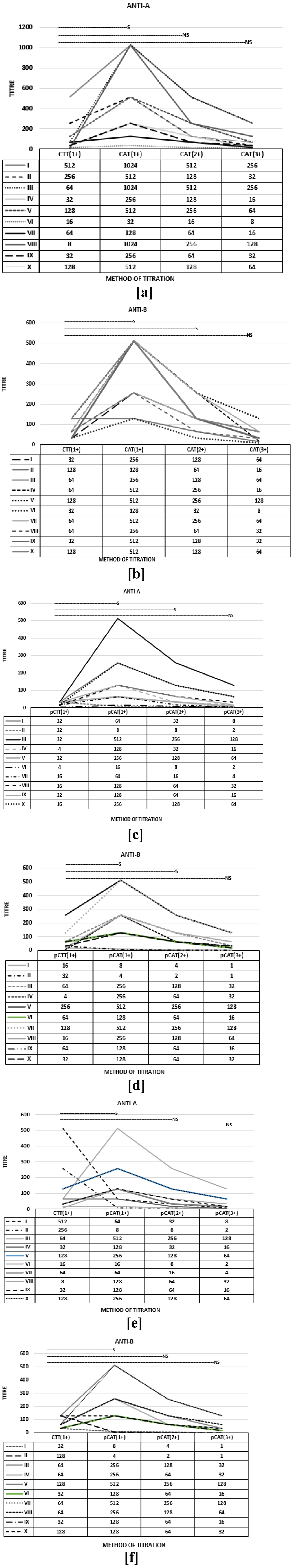 Figure 3: Comparison of IgG titers performed by CTT at 1+ end point and CAT at 1+/2+/3+ end points for 10 samples. a) Pre DTT treatment CTT anti-A titers compared with Pre DTT treatment CAT anti-A titres; b) Pre DTT treatment CTT anti-B titers compared with Pre DTT treatment CAT anti-B titres; c) Post DTT treatment CTT anti-A titers compared with Post DTT treatment CAT anti-A titres; d) Post DTT treatment CTT anti-B titers compared with Post DTT treatment CAT anti-B titres; e) Pre DTT treatment CTT anti-A titers compared with Post DTT treatment CAT anti-A titres; f) Pre DTT treatment CTT anti-B titers compared with Post DTT treatment CAT anti-A titres. Wilcoxon signed rank test was used for calculating significance using p < 0.05. S indicates significant and NS indicates not significant.
View Figure 3
Figure 3: Comparison of IgG titers performed by CTT at 1+ end point and CAT at 1+/2+/3+ end points for 10 samples. a) Pre DTT treatment CTT anti-A titers compared with Pre DTT treatment CAT anti-A titres; b) Pre DTT treatment CTT anti-B titers compared with Pre DTT treatment CAT anti-B titres; c) Post DTT treatment CTT anti-A titers compared with Post DTT treatment CAT anti-A titres; d) Post DTT treatment CTT anti-B titers compared with Post DTT treatment CAT anti-B titres; e) Pre DTT treatment CTT anti-A titers compared with Post DTT treatment CAT anti-A titres; f) Pre DTT treatment CTT anti-B titers compared with Post DTT treatment CAT anti-A titres. Wilcoxon signed rank test was used for calculating significance using p < 0.05. S indicates significant and NS indicates not significant.
View Figure 3
ABO isoagglutinins play a major role in outcome of ABO incompatible grafts in case of solid organ as well as hematopoietic stem cell transplant [27-29]. Since the concentration of these antibodies regulate the immune reactions related to transfusion and transplantation, their measurement and preoperative as well as postoperative monitoring is very important. O blood group individuals are known to possess more IgG ABO isoagglutinins as compared to A and B blood groups [22]. In the present study, IgG titers measured by CAT and CTT both were higher than IgG titers. When comparing 1+ reaction strength, both IgM and IgG titers were found to be more when measured by CAT as compared to CTT. IgG antibodies are believed to play a crucial role in graft outcome and hence IgG titre estimation with use of DTT has been recommended [6,12]. DTT inactivates IgM antibodies with minimal effect on IgG antibodies [6,9].
Results of the present study show that results obtained by CAT have less inter-observer variation. CAT has been recommended for various immunohematological investigations due to its sensitivity. However, for antibody level determination, it is not explored well [18,29]. There was significant difference between the antibody titer readings obtained by CTT and CAT and those obtained by DTT treatment with CAT results beings higher in general compared to CTT results. We compared antibody titer obtained using DTT treated and untreated samples. A reduction in anti-A and anti-B titers was observed by DTT treatment both by CTT as well as CAT. However, the reduction in median titers was more for CTT when compared to CAT. This indicates that presence of IgM antibodies in samples leads to overestimation of IgG titers which can directly impact management of ABO incompatible transplant recipients and this effect is more in CTT. Without the use of DTT, there is estimation of total antibody titer. Nayak, et al. compared results of 50 samples and concluded that there was poor agreement between IgG titers performed by CAT and CTT [30]. Matsuura, et al. enrolled 10 individuals with blood group O and performed antibody titration simultaneously using CTT and automated CAT. They used DTT treated plasma for automated titer estimation by CAT to define the cut-off value in antibody titration and found 45% concordance and a significant positive correlation between CTT and automated CAT with weak strength of reaction. They recommended use of DTT for titer estimation by automated CAT [31]. In the present study, 1+ end point for CTT was compared for agreement of results with 1+, 2+ and 3+ end points of CAT with and without DTT treated plasma. There was 43.4% and 40.3% agreement of the anti-A IgM and IgG titers between CAT (2+ strength) and CTT (1+ strength) respectively. There was 43.6% and 32% agreement of the anti-B IgM and IgG titers between CAT (2+ strength) and CTT (1+ strength) respectively. There was 3.5% and 11.5% agreement of the anti-A IgM and IgG titers between pCAT (3+ strength) and CTT (1+ strength) respectively. There was 0.8% and 10.9% agreement of the anti-B IgM and IgG titers between pCAT (3+ strength) and CTT (1+ strength) respectively. There was 15.4% and 21.3% agreement of the anti-A IgM and IgG titers between pCAT (2+ strength) and CTT (1+ strength) respectively. There was 14.8% and 30.9% agreement of the anti-B IgM and IgG titers between pCAT (2+ strength) and CTT (1+ strength) respectively. There was 24.2% and 28.7% agreement of the anti-A IgM and IgG titers between pCAT (1+ strength) and CTT (1+ strength) respectively. There was 30.2% and 17.5% agreement of the anti-B IgM and IgG titers between pCAT (1+ strength) and CTT (1+ strength) respectively. When correlation was calculated, in the present study, IgM and IgG measurement of anti-A and anti-B showed strong correlation between CTT (1+ strength) and CAT (1+, 2+ strengths). Similarly, IgM and IgG titers for both anti-A and anti-B showed strong correlation between CTT (1+ strength) and pCAT (2+ strength). Though agreement of results between CTT and CAT (2+) were found to be better than CTT (1+) and pCAT (2+); it is difficult to determine which method of titration is superior.
Park, et al. tested 60 individuals with blood group O for titer estimation by CTT and CAT, with and without DTT treatment. They observed that higher median titers of anti-A and anti-B were obtained by CAT than CTT. Median titers were found to be higher in CAT as compared to pCAT titers. They concluded from their study that results obtained by CAT with or without DTT treatment were more sensitive than CTT for group O individuals [32]. Shim, et al. compared three methods of antibody titration using 40 samples and found that found that the median IgM and IgG titres were higher by CAT with the agreement being better for IgM compared to IgG [33]. In the present study, median titers were determined separately for IgM and IgG for anti-A and anti-B as illustrated in Figure 2. Median titers observed by pCAT were mostly higher than those obtained by CTT.
Strengths of this study include a robust sample size and performing results in duplicate by two separate individuals. This is the first study which assesses the effect of DTT on anti-A and anti-B titers in more than 2000 O blood group individuals with use of two different methods; CAT and CTT. Limitations of this study include inability to assess clinical impact of titration performed before and after DTT treatment.
The findings of the present study suggest that there is significant difference between antibody titers estimated using DTT treated and untreated plasma. It is evident that DTT treatment leads to significant reduction in titers both by CTT as well as CAT. CAT results were found to more sensitive than CTT titer results. The authors strongly recommend use of DTT for ABO isoagglutinin titer estimation. However, clinical impact of these titer results need to be performed to find an appropriate method for titer estimation.
The authors would like to acknowledge all the personnel, study participants and Mr. Manish K. Singh, biostatistician for his invaluable help with statistical analysis. PKP was the guarantor for the study. Concept, design and intellectual content was defined by PKP, DS and SR. Literature search, experimental studies, data acquisition, data analysis and statistical analysis was done by DS, SR and SK. Statistical analysis was performed by DS, SR. Manuscript was prepared by DS and SR. PKP reviewed the manuscript.
The authors declare no conflict of interest.
None.