Food is important to life and the continuous exposure to food throughout an individual's lifetime renders diet the most important environmental factor challenging the biological system. Only few studies exist for evaluations of the toxicological effects of adulterated palm oil on biochemical parameters. This study was undertaken to evaluate the expression of the activity of inflammatory enzymes (TNF-α, MCP-1), antioxidant enzymes (GPx-1, CAT) and functional markers (EPO, ALB, CRIM) in liver, kidney and testicular tissues of albino Rats and to check for probable weight difference in treated animals before and after treatment. 25 albino rats were divided into 5 groups and treated as thus; group I (control), groups II and III (1 ml/kg of unadulterated and adulterated palm oil respectively), groups IV and V (50 mg/kg Sudan III and IV respectively) for 28 days. Gene expression levels were evaluated using reverse transcriptase and polymerase chain reaction protocols. The expression of inflammatory markers: Tumor necrosis factor α (TNFα), monocyte chemoattractant protein-1 (MCP-1) and oxidative stress markers catalase: (CAT), glutathione peroxidase-1 (GPx-1) were significantly up regulated (p < 0.05) in the liver, kidney and testes with the expression of functional markers: Albumin (ALB), erythropoietin (EPO), calcium responsive transcription factor (CRIM) significantly (p < 0.05) down regulated in groups III, IV and IV when compared to control groups. No weight gain was observed in treated animals before and after treatment. Ultimately, Sudan dyes are able to induce production of ROS which has been implicated in several disease conditions thus not safe for consumption.
Antioxidants, Inflammatory, Functional marker, Gene expression
The Oil palm (Elaeisguineensis) is a monocotyledonous plant belonging to the palm family Arecaceae. It is a monoecious specie known to produce unisexual male and female in florescences in an alternating cycle [1]. The fruit produces two distinct types of oil: Orange-red crude palm oil which is extracted from the mesocarp and brownish yellow crude palm kernel oil extracted from the seeds (kernel). Food fraud is currently a persistent global problem with advancing technology and no food commodity is left out as in the case of palm oil [2]. Food Adulteration can be defined as a process of lowering the quality of food by intentional or unintentional substitution of food with some inferior foreign particle or by removal of some value added food substitute from main food item [3]. Toxicity may however relate to the routes of exposure, dose and personal characteristics such as age and health conditions may affect the individual's susceptibility [4].
The most widely reported adulterant of palm oil are Sudan III and IV azo dyes, they are being added to palm oil to improve the colour and make it more appealing to consumers [2,5,6]. Azo Sudan dyes are synthetic organic colorants considered illegal dyes, mainly because of its probable harmful effect over a long period of time [5]. The azo groups are generally connected to benzene and naphthalene rings, but can also be attached to aromatic heterocycles or enolizable aliphatic groups. The color of the azo dyes is determined by the azo bonds and their associated chromophores and auxochromes [7,8].
Addition of these dyes (Sudan I, II, III, IV) into foodstuffs contravenes EU and USA legislation and is currently banned in almost all countries. Sudan dyes are illegally used to adulterate food and had been labelled group 3 carcinogens due to the induction of some types of cancer related to bladder and liver in animals and the incidence of their abuse is reported in palm oil samples worldwide [9-12]. The potential for mutagenicity of azo dyes depends on the ability of the molecule to produce aromatic amines which are active and semiquinone radicals. Semiquinone radicals on further degradation produce superoxide, hydroxyl radicals and H2O2 [13]. This action is influenced by the structural relationship, which results in the breakage of the azo linkage leading to oxidation of the primary aromatic amine which has been liberated [14].
Inflammation is a local response of living vascularized tissues as a way of protecting tissues from infection, injury or disease. The inflammatory response begins with the production and release of chemical agents by cells in the infected, injured or diseased tissue [15]. These agents cause redness, swelling, pain, heat and loss of function. TNF is a multifunctional cytokine, a pro-inflammatory cytokine secreted by inflammatory cells involved in inflammation associated with carcinogens. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages. Both CCL2 and its receptor CCR2 have been demonstrated to be induced and involved in various diseases [16]. The targeting of inflammatory mediators (chemokines and cytokines, such as TNF-α and MCP-1), key transcription factors involved in inflammation (such as NFKB and STAT3) or inflammatory cells decreases the incidence and spread of cancer. Superoxide radicals (O2•-), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), and singlet oxygen (1O2) are commonly defined reactive oxygen species (ROS); they are generated as metabolic by-products by biological systems. Oxidative stress however arises when there is an imbalance between production and accumulation of oxygen reactive species (ROS) in cells and tissues and the ability of a biological system to detoxify these reactive products [17]. A major challenge associated with the use of adulterants in palm oil is that the adulterants have not undergone adequate research and the degree of health hazards they can pose to humans when consumed not well established [13,18].
• Test for adulteration of palm oil: Palm oil samples were left to stand on the bench for six (6) months in transparent pet bottles, red dyes were seen settled at the base of palm oil containing adulterants.
• Chemical test: The method used by Nwachoko and Fortune [19] was employed to check for the presence of adulterant-Sudan dye in samples of palm oil. This analysis was done using petroleum spirit and concentrations of hydrochloric acid/ water (1:1). To 5 ml of each oil sample in different test tubes, 15 ml of petroleum ether was added followed by the 5 ml hydrochloric acid to different test tubes. Different shades of yellow were observed at the top and a clear/colorless base indicating the absence of adulterants while samples containing adulterants showed a reddish yellow top and a reddish clear base.
25 male albino rats were weighing 111g-198g were assigned into 5 groups (I, II, III, IV, V). Five animals in each cage, they were acclimatized to their environment and diet for 7 days. Groups II, III, IV and V were the test groups. Group I was the control group. Groups II and III were given 1 ml/kg of unadulterated palm oil and adulterated respectively [19]. Groups IV and V were given 50 mg/kg Sudan III and Sudan IV respectively [20].
Total RNA isolation: Total RNA was isolated from whole tissues following a method described by [21].
PCR amplification and agarose gel electrophoresis: PCR amplification for the determination of genes (Primer3 software).
Figure 1, Figure 2, Figure 3 and Figure 4.
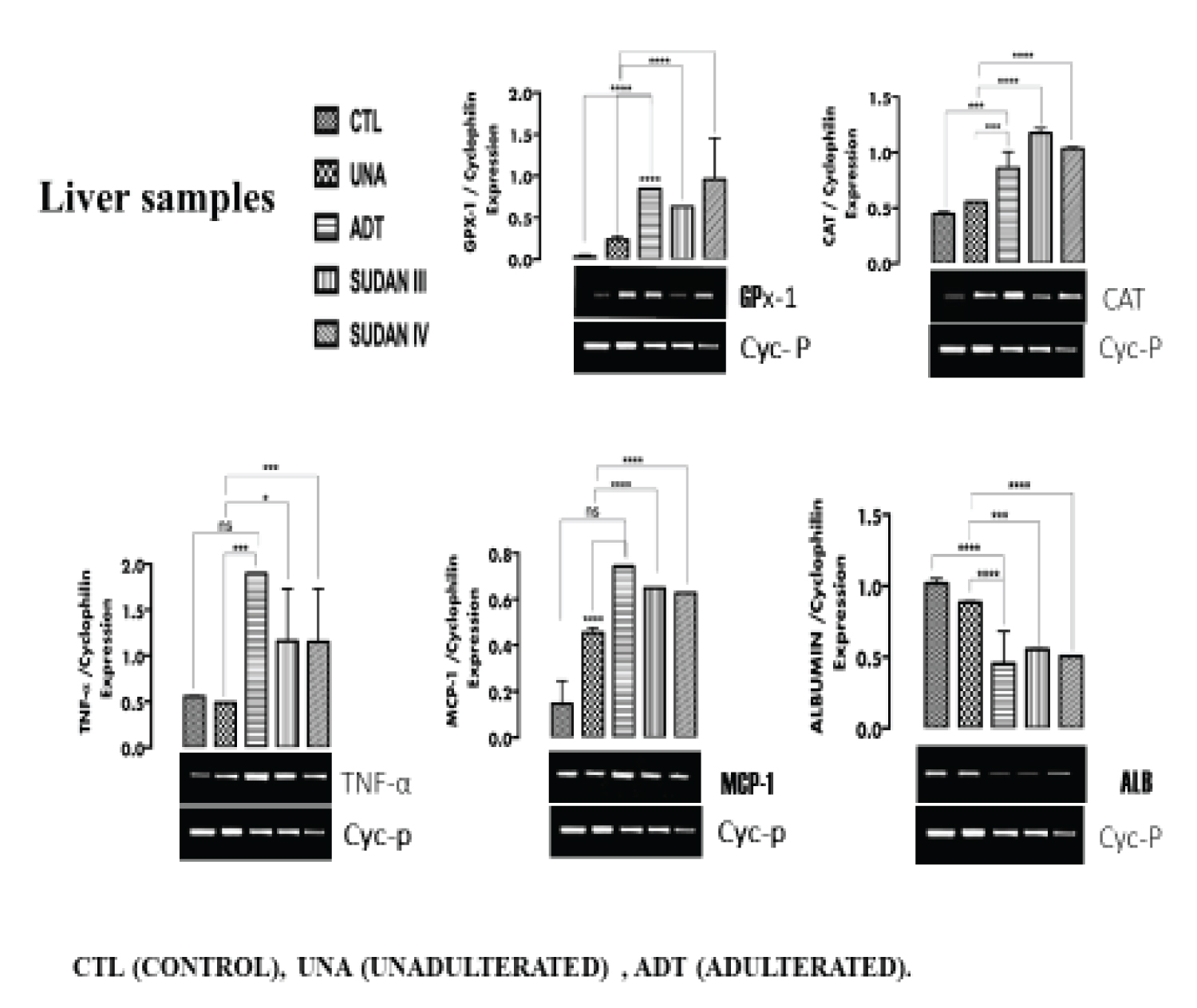 Figure 1: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (ALB) in the Liver tissue. View Figure 1
Figure 1: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (ALB) in the Liver tissue. View Figure 1
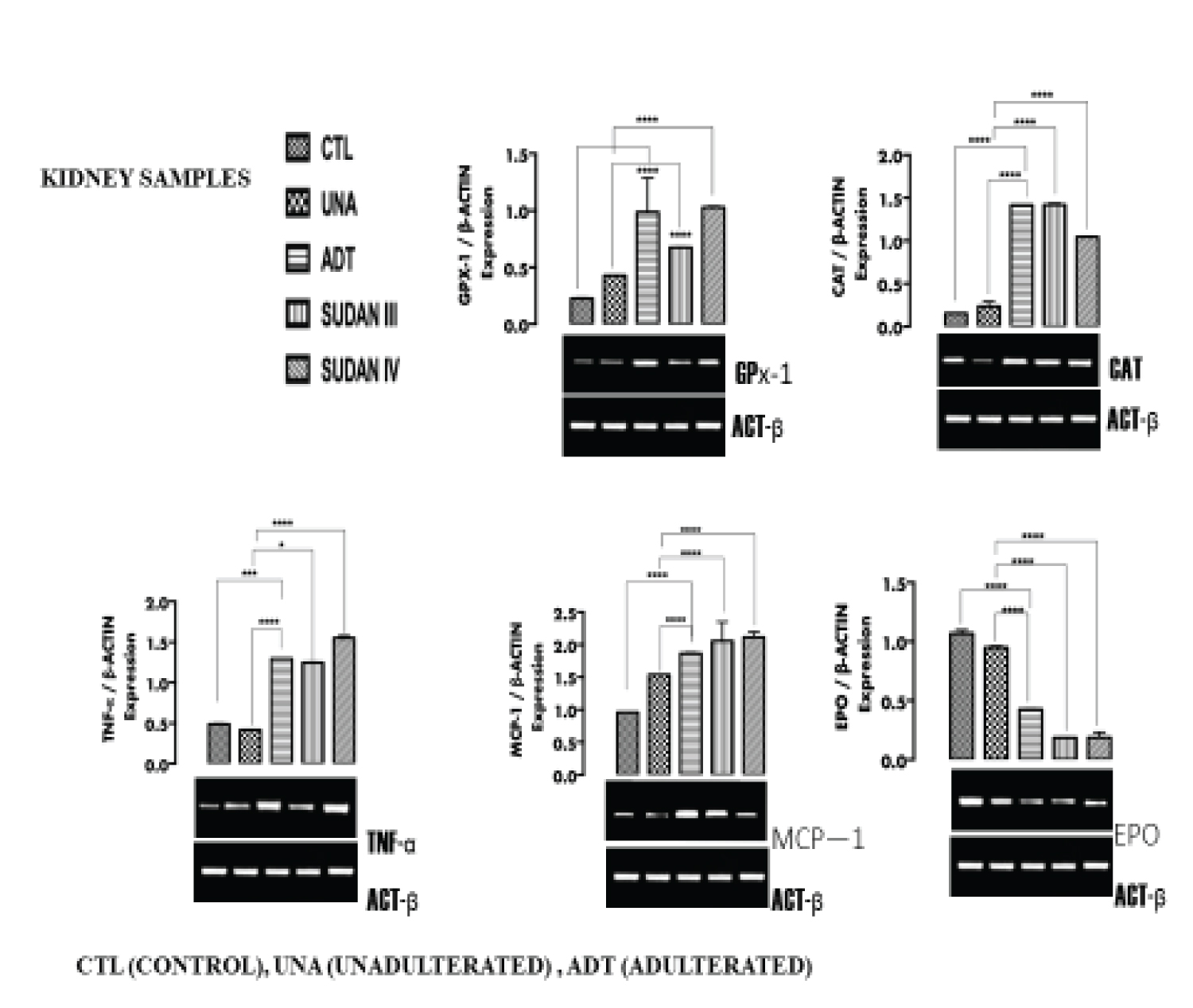 Figure 2: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (EPO) in the Kidney tissue. View Figure 2
Figure 2: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (EPO) in the Kidney tissue. View Figure 2
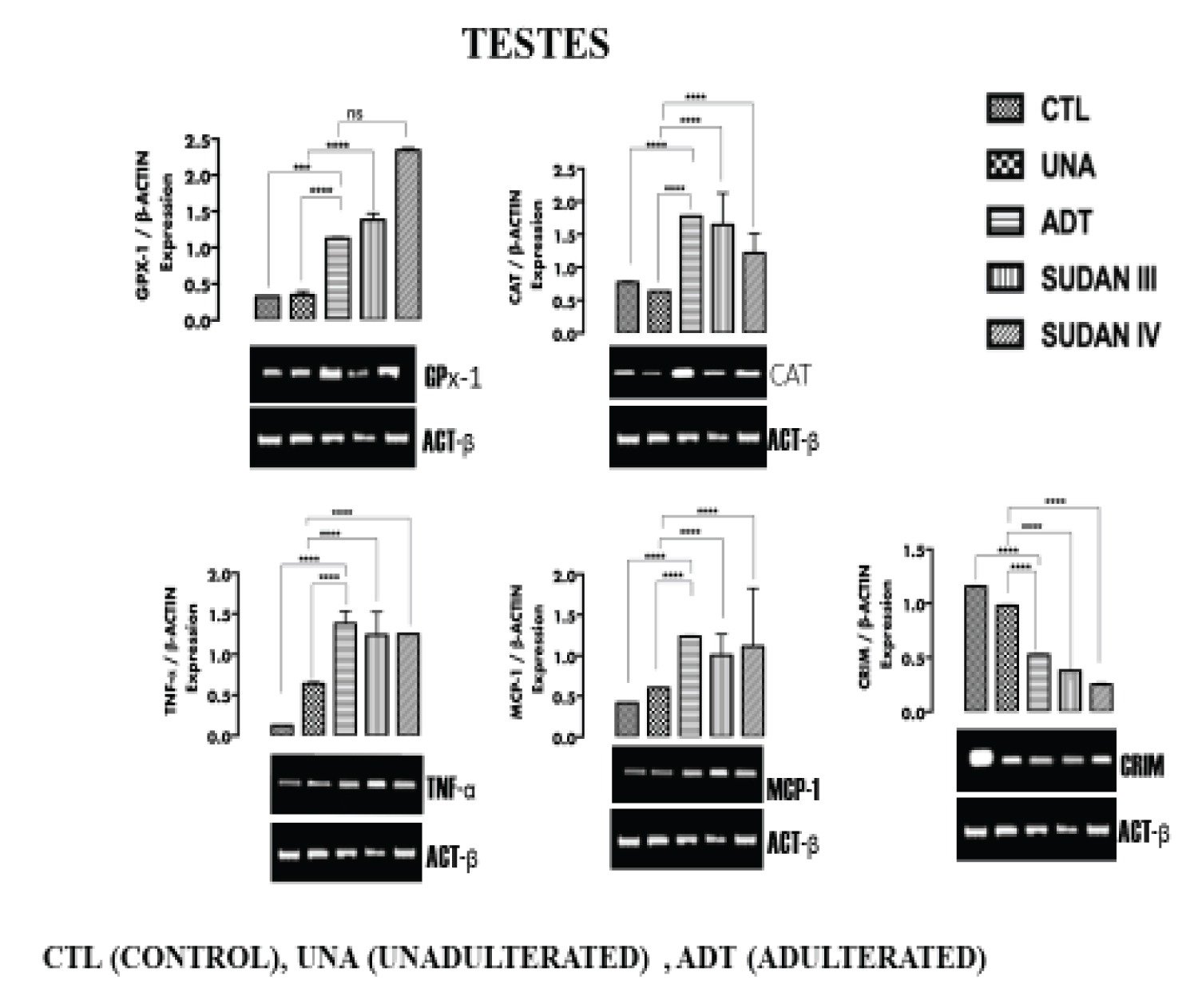 Figure 3: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (CRIM) in the Testicular tissue. View Figure 3
Figure 3: The expression of inflammatory markers (TNF-α, MCP-1), oxidative stress markers (CAT, GPx-1) and functional marker (CRIM) in the Testicular tissue. View Figure 3
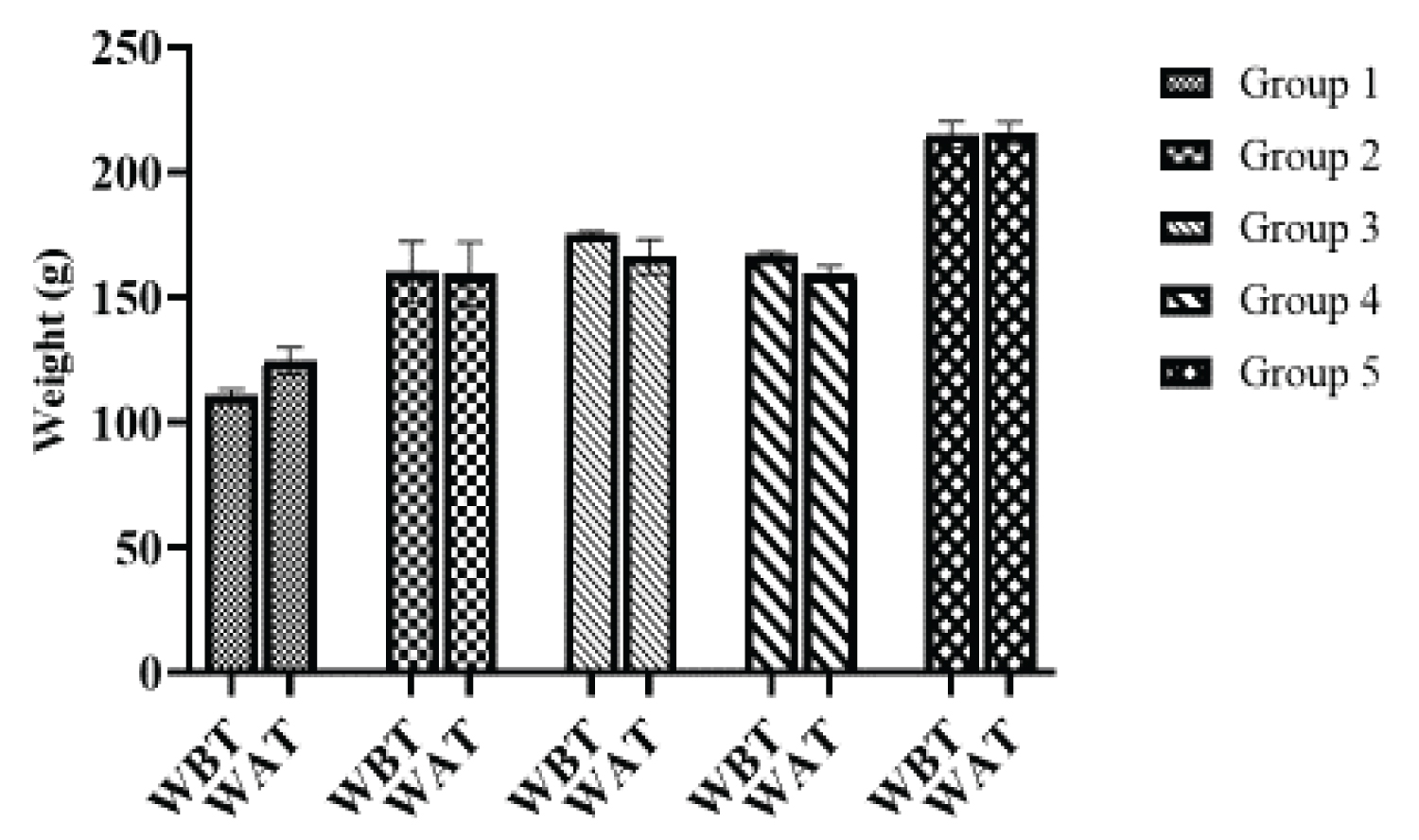 Figure 4: Average weight of animals before treatment (WBT) and after (WAT) administration of adulterated palm oil and Sudan dyes. View Figure 4
Figure 4: Average weight of animals before treatment (WBT) and after (WAT) administration of adulterated palm oil and Sudan dyes. View Figure 4
From Figure 1 (liver), Figure 2 (Kidney), Figure 3 (testicular tissue), a significant (p < 0.05) upregulation in the expression of antioxidant enzymes Catalase (CAT) and Glutathione peroxidase (GPx-1) in the liver, kidney and testicular tissues of rats administered adulterated palm oil, Sudan III and Sudan IV dyes (groups III, IV and V respectively) were observed when compared to the control groups (I and II). In line with this study Shaista, et al. [22] reported alterations in the activities of antioxidant enzymes Catalase (with an increase by more than 2.5 folds) when Drosophila melanogaster was exposed to textile dyes. Samar, et al. [23] also reported upregulation of oxidative stress markers in male rats exposed to Sudan II azo dye in dose-dependent manner.
The antioxidant network acts as a defense mechanism against stress, antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, acts as free radical scavengers [24,25]. Recent studies have pointed to the ability of azo dyes to induce oxidative stress [26,27]. The initial step of azo dye metabolism is reduction of the azo bond catalyzed by azo reductase to form aromatic amines and semiquinone radicals [28,29]. Semiquinone radicals on further degradation produce superoxide, hydroxyl radicals and H2O2. This leads to the reduction of the body's defense system, thereby generating a variety of oxidative stress related conditions [30]. The reduction of azo bonds is important for its toxicity, mutagenicity and carcinogenicity [31]. The mutagenic, carcinogenic and toxic effects of azo dyes stem from the direct effect of the dye or indirectly from the reductive biotransformation of the azo bond during its metabolism [32].
The upregulation of CAT and GPx-1 observed in the tissues is in response to reactive species which potentially induce oxidative stress.
Figure I (liver), Figure 2 (Kidney) and Figure 3 (Testicular tissue) a significant (p < 0.05) upregulation in the expression of Tumor necrosis factor- α (TNF-α) and Monocyte chemoattractant protein-1(MCP-1) in the liver, kidney and testicular tissues of rats in group III, IV and V were observed when compared to the control groups (I and II). In tandem with this study, Bianca, et al. [26] reported the ability of azo dye to induce proinflammatory effects, also line with this study, Yasmina, et al. [27] reported marked upregulation of inflammatory markers (IL-1β, IL-6, and IL-10) on long term exposure to tartrazine in rats. Leo, et al. [33] reported the ability of azo dyes to elicit proinflammatory effects in samples of food products. Ives, [34] reported up regulation in the expression of TNF-α in in vitro cell culture.
Azo dye has been said to pose health risks and exert negative effects on the kidney and nervous system [26]. TNF-α and MCP-1 are usually elevated in response to inflammation, this inflammation in tissues has been attributed to aromatic amines produced from the degradation of azo dyes [27]. Recent advances in the field of cardiovascular medicine have reiterated the involvement of inflammation in the formation of atherosclerotic plaque. It has been shown that inflammatory signals at the site of plaque initiation attract monocytes from the circulation into the vascular wall to form lipid-laden foam cells and promote smooth muscle cell proliferation, resulting in plaque progression [35]. Exposure to Sudan dye adulterated palm oil can actually predispose an individual to cardiovascular disease. The induction of inflammation by azo dyes also allows leukocytes (white blood cells), which are normally filtered out of the joint, to invade the joint space. The inflamed synovial membrane and the leukocytes release free radicals, cytokines, and prostaglandins, all of which are potentially damaging to the articular cartilage causing joint pain [26].
Figure 1 (liver), Figure 2 (Kidney) and Figure 3 (Testicular tissue) shows significant (p < 0.05) downregulation in the expression of Albumin (ALB), Erythropoietin (EPO) and calcium responsive transcription factor (CRIM) in the liver, kidney and testicular tissues of rats in groups III, IV and V when compared with the control groups (I and II). In line with this study, Dawei, et al. [36] reported a damage to Bovine serum albumin (BSA) when there was an interaction with Sudan II and IV dyes. Structural analysis shows that Sudan dyes can interact with Albumin (and other proteins) at its hydrophobic pocket via Van der Waals forces, forming a bond with the protein and causing alteration [36]. The downregulation in the expression of ALB, EPO and CRIM in the liver, kidney and testes is an indication of probable gradual loss of function of the organs.
From Figure 4, no significant (p > 0.05) weight gain was observed at a dose of 50 mg/kg body weight in treated animals before and after treatment. In line with this study, Imafidon and Okunrobo, [37] reported no weight gain in albino rats administered Sudan IV dye but contrary to the findings in this study, Oparaocha, et al. [20] reported weight gain at a dose of 100 mg/kg and 150 mg/kg body weight on exposure to Sudan dye. The no observed weight gain in this study may be as a result of the low dose administered to the rats (50 mg/kg). It can be said that at that dose, weight gain was not induced but this does not rule out the fact that Sudan dyes may induce weight gain.
This study was able to establish that azo dye adulterated palm oil can induce oxidative stress, inflammation and also cause organ malfunction, all of which has been implicated in the pathogenesis of a number of diseases which includes cardiovascular disease, aging, joint pain and some types of cancer.
Not applicable.
Not applicable.
All animal experiments were approved by the quality control unit of the university.
Not applicable.
The authors declare that there are no conflicts of interest.
Data generated as part of this study is available upon request from the corresponding author.
All authors provide consent for publication.