Thalis 112 EC is the trade name of a binary insecticide (Emamectin benzoate 48 g/L, Acetamiprid 64 g/L) used in cotton growing in Benin. These compounds can lead to extensive side effects on the inhabitants living in that exposed area. The present study aims to evaluate the effects of chronic exposure to this pesticide on the immune status of Clarias gariepinus. Thus, 300 juveniles of C. gariepinus (average weight 30.0 ± 2.64g) were exposed in glass aquariums to sub-lethal concentrations of Thalis (T0 = 0.0; T1= 0.03; T2 = 0.06; T3 = 0.12; T4 = 0.25 and T5 = 0.49 ppm) for 28 days. The stocking density is 14 fish per tank filled with 25 L of test solution. The test took place in a semi-static system with 50% renewal of the test solutions every 48 h. It appears from the study that the presence of Thalis leads to an increase in WBC in C. gariepinus. As for Total immunoglobulin, the presence of a low concentration (T1) of the pesticide leads to an increase followed by a decrease as the concentration increases. However, Thalis did not induce a significant effect on PCV, Hb and Erythrocyte constants (MCV, MCH and MCHC), even though Hb and these Erythrocyte constants showed an increasing trend. The RBC also showed an increasing trend. This haematological profile obtained with C. gariepinus exposed to Thalis is probably linked to the binarity of this toxin made up of molecules from various families whose modes of action are different. It is concluded that indiscriminate use of such pesticides poses a noxious threat to non-target organisms, harm the ecosystems and jeopardizes human health.
Thalis 112 EC, Chronic effects, Hematology, Clarias gariepinus
At the current state of agricultural practices, conventional cotton is the one produced mainly in Benin and which holds the sustained attention of the public authorities [1-5]. Biological cotton has been still in the trial stage for years and occupies only 0.6% of national production [6]. Conventional cotton production involves the use of mineral fertilizers for soil fertilization and chemical pesticides for weed control and phytosanitary treatment of plants [7]. Thus, in order to achieve the dual objective of high seed cotton yield and very good quality lint cotton, insecticides occupy a prominent place in the technical itinerary of cotton growing in Benin [1]. The insecticides are used as binary acaricides or aphicides [3-5,7]. The increasing resistance of the targeted pests and, consequently, the rapid loss of efficacy, has led to a massive use of this control strategy [8]. At least six treatments are recommended to fight against these cotton pests. The first occurs on the 50th day after sowing and two treatments must be separated by 14 days [1]. Among the binary insecticides used in the largest cotton basin of northern Benin since the 2016-2017 cotton campaign are Thalis 112 EC, Vizir C 92 EC, Pyrinex Quick 212 EC, Pyro FTE 472 EC, etc [9]. Thalis, based of Emamectin benzoate (48 g/L) and Acetamiprid (64 g/L), is an aphycidal binary used in the first and 2nd window to fight against stinging-sucking insects and the first generation carpophagous moths Helicoverpa armigera [2]. In view of the volume of this toxicant used in cotton fields, this insecticide currently occupies a place of choice. Several studies had already revealed that, in the Benin cotton basin, the doses of pesticides recommended for the treatment of crops are not necessarily those practiced by farmers [1,10]. These growers increase the recommended amounts of pesticides at will [1]. All these chemical molecules released into the environment seep into the ground or run off and reach aquatic ecosystems as their final receptacle [11], thus making the species that live there vulnerable. Until recently, the adverse effects of pesticides and their residues on non-target organisms have not been seriously considered in Benin. This situation is worrying, especially with the phenomenon of climate change where the toxicity of several chemical pollutants is increasing as a result of global warming [7].
Emamectin benzoate belongs to the Avermectin family [12]. Clarias gariepinus juveniles are highly sensitive to Ivermectin, with a LC50 of 15 μg/L under static conditions [13]. Studies found that Emamectin benzoate altered the blood factors in Labeo rohita [14], in Oreochromis niloticus [15] and in C. gariepinus [13]. Acetamiprid is a molecule of the first generation of Neonicotinoid family [16]. Its 96-h LC50 was determined at 182.9 ppm for O. niloticus [8] and 265.7 ppm for C. gariepinus juveniles [17]. Acetamiprid is found to be hemotoxic in L. rohita [18], in Cyprinus capio [19] and in Cirrhinus mrigala [20].
The individual effect of the active substances is different from that when they are mixed [8,17]. The overall objective of this study is to determine, under local laboratory conditions, the effects of the combined toxicity of Emamectin benzoate and Acetamiprid on blood profile in C. gariepinus, an important species of aquatic ecosystems receiving insecticides and herbicides from the cotton basin of northern Benin. This involves determining the effects of Thalis on immune parameters in C. gariepinus.
Three hundred healthy freshwater fish C. gariepinus having average weight 30.0 ± 2.64g was collected from fish Farm Agro-business complex located in Parakou (Benin) which is relatively free from pollutants (50% females and 50% males). Fish were transported to the Research Laboratory in Aquaculture and Aquatic Ecotoxicology (LaRAEAq), University of Parakou, within well packed polythene bags containing aerated water.
The fish were acclimatized in laboratory environment for 21 days before beginning of the experiment and fedthice daily with commercial dry feed GOUESSANT pellets of 2 mm (Crude protein 46%). The aquariums were connected with constant system of aeration with a 12 ± 1:12 ± 1 light-dark cycle. Temperature was retained from 26.2 to 30.0 °C and pH from 7.0 to 7.8. During this period, the stock water was changed once every other day to prevent the accumulation of decaying food particles and waste metabolites.
The insecticide Thalis 112 EC based of Emamectin benzoate (48 g/L) and Acetamiprid (64 g/L) commonly used by farmers in the cotton basin of Benin was purchased from "Societe de Distribution des Intrants" for this study. This binary insecticide is in viscous yellow liquid form. The test solutions were obtained by mixing the product directly with dechlorinated tap water, as it is done in a farming environment. All working stock solutions were made immediately prior to the tests.
The experiment was conducted according to OECD Guidelines 215 with some minor adaptations. Based on the value of 96 h LC50 = 4.9 ppm (Data not yet published), five concentrations i.e. T1 = 0.03 ppm (0.6% 96 h LC50), T2 = 0.06 ppm (1.2% 96 h LC50), T3 = 0.12 ppm (2.5% 96 h LC50), T4 = 0.25 ppm (5% 96 h LC50) and T5 = 0.49 ppm (10% 96 h LC50) were selected for this chronic toxicity test. No pesticides were added in control group T0. Eighteen aquaria (Volume = 30 L) were used for these six conditions with three replicates per treatment. Fourteen fish were randomly distributed in each glass tank containing 25L test solutions. The test was carried out under semi-static conditions with 50% renewal of the test solutions every 48 h. During the test, the fish were fed to apparent satiety 3 times a day with an artificial food GOUESSANT pellets of 3 mm (Crude protein 46%). The exposure lasted 28 days. The aquariums were connected with constant system of aeration with a 12 ± 1:12 ± 1 light-dark cycle. The physico-chemical parameters such as the pH and the temperature of the water were recorded every 48 h after and before renewal of the test solutions. The aquariums were covered with netting in order to prevent the fish from jumping from one aquarium to another. No mortality was observed during this test.
Blood was collected from the caudal vein using a 1 mL disposable syringe. Fishes were first treated with 3% salt solution to reduce stress and then anesthetized with clove oil (0.4 mLL-1 of water) before collection of blood. Blood samples were collected at the end of 28 days of exposure to Thalis on 6 fish per aquarium (3 males and 3 females). The collected blood was stored in EDTA vials for the complete blood cell count, and 1.5 mL eppendorf tube for serum collection.
Haematological parameters such as Heamatocrit, Total immunoglobulins (IgT) and Hemogram or Complete blood count were analyzed. The Heamatocrit level is the ratio between the cell volume (Red blood cells) and the Total blood volume expressed as a percentage. The Heamatocrit was calculated from the values after reading a ruler graduated in centimeters of the length of the Red blood cell and that occupied by the whole blood obtained after centrifugation at 3000 RPM for 5 min of the blood samples. Heamatocrit is also called Packed cell volume (PCV). The level of immunoglobulins in the serum was assessed by spectrophotometry, as previously established by Milla, et al. [21]. The protein assay was carried out using the technique developed by Bradford, based on the absorption of Coomassie blue G250 on proteins in an acid environment. The quantity of Total immunoglobulins is the difference between the quantity of protein in the serum before and after precipitation of large proteins. The Hemogram was carried out using a flow cytometer (SYSMEX XP 300), the results of which were supplemented by blood smears. This study revealed the different leukocyte cells (Basophil, Neutrophil, Eosinophil, etc.), Total count red blood cells (RBC), White blood cells (WBC), Hemoglobin level (Hb) and Erythrocyte constants such as mean Corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC) and Mean corpuscular haemoglobin (MCH).
The experimental unit is the aquarium. The results were expressed as mean ± standard deviation of the mean. Before analyzing the data, the Shapiro-Wilknormality test was performed and if it was normal, the ANOVA test was used. For a two-by-two comparison of means, Fisher’s LSD test was used under STATISTICA software version 6.P value of 0.05 or less was considered significant.
Table 1 presents the mean temperature and pH values measured during the experiment. It appears that there is no significant difference between the values either before or after renewal of the test solutions. The values recorded for the temperature vary between 27.02 (T4) and 27.24 °C (T0) before emptying, then 26.97 (T5) and 27.18 °C (T0) after the water renewal. For the pH, the minimum and maximum values are 6.84 (T5) and 6.92 (T0) before emptying, then 6.71 (T5) and 6.76 (T0 and T1) after emptying.
Table 1: Physico-chemical quality of test solutions. View Table 1
Heamatocrit (PCV): In this study, the PCV level remained statistically similar (p > 0.05) during exposure from one treatment to another and according to sex. This rate oscillates between 25.04% (T3) and 31.08% (T4) in females on the one hand, and 32.11% (T4) and 36.5% (T2) in males on the other hand (Figure 1).
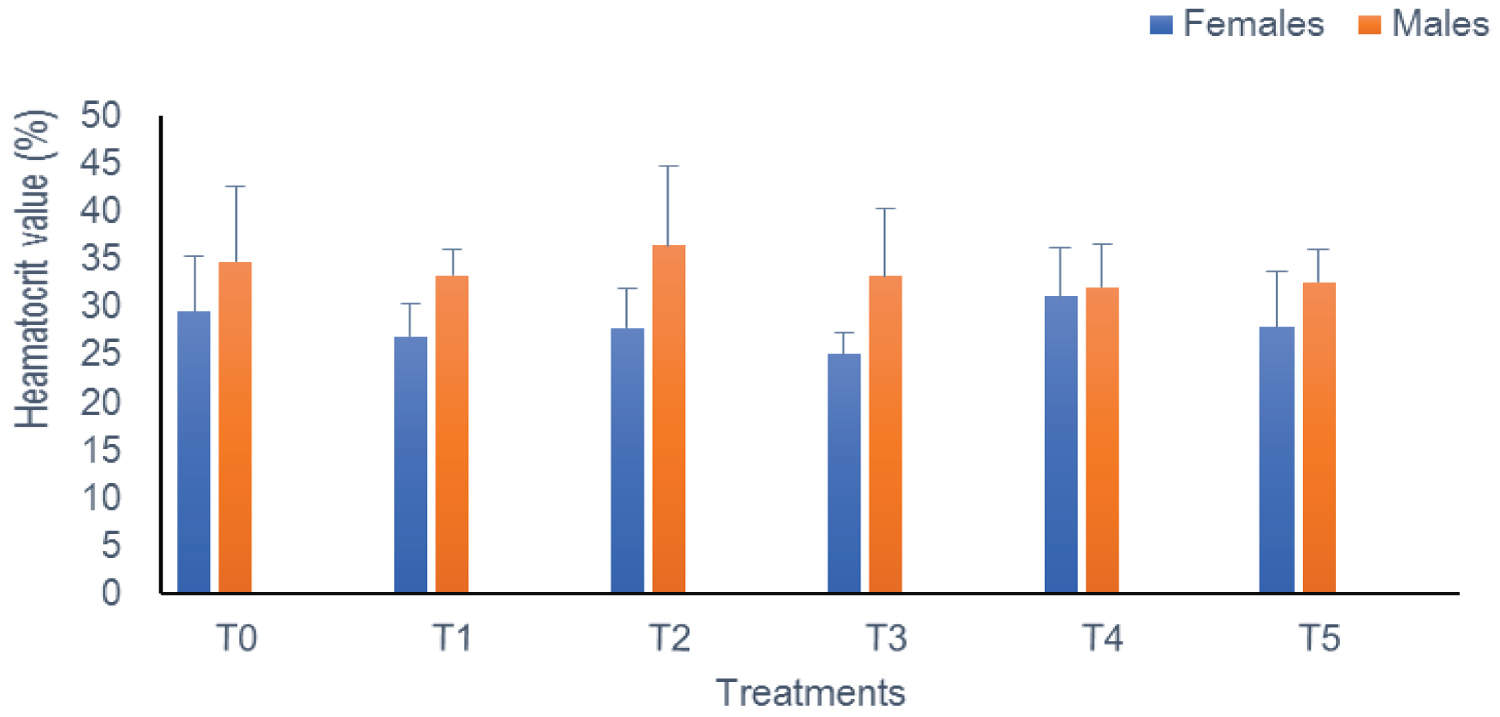 Figure 1: The heamatocrit of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 1
Figure 1: The heamatocrit of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 1
Total immunoglobulins (IgT): The results of the effect of sub-lethal concentrations of Thalis on IgT in C. gariepinus are shown in Figure 2. These results indicate a significant difference (p < 0.0001) between the treatments according to sex. In males, there is an increase in the IgT of T1 compared to the control (T0 = 163.86 mg/mL and T1 = 193.23 mg/mL) as in females (T0 = 148.52 mg/mL and T1 = 178.50 mg/mL). These IgT values gradually decrease to 84.14 mg/mL in males T4 and 99.28 mg/mL in females T4.
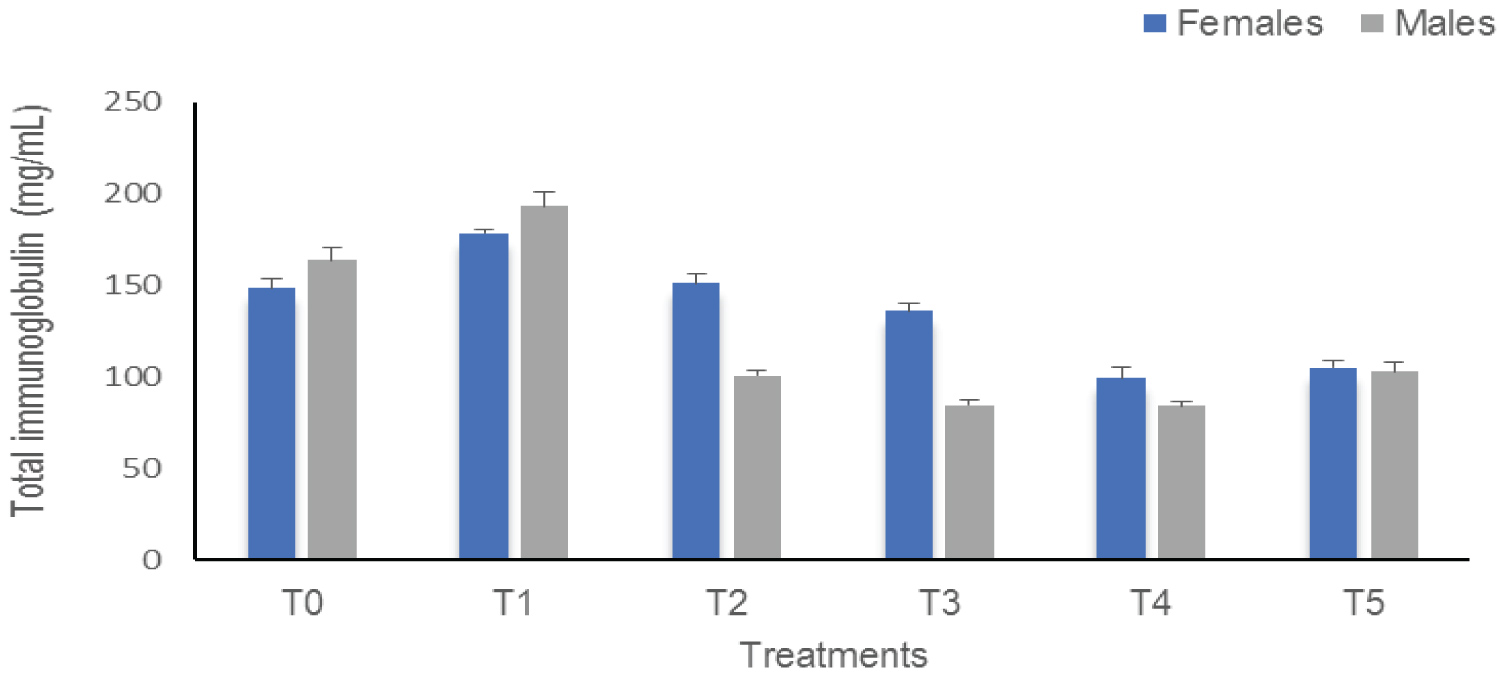 Figure 2: The Total immunoglobulins of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD.
View Figure 2
Figure 2: The Total immunoglobulins of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD.
View Figure 2
Differential leukocyte count: The different leucocyte cells observed under an optical microscope (x100 objective) on the blood smear slides are represented in Figure 3 below.
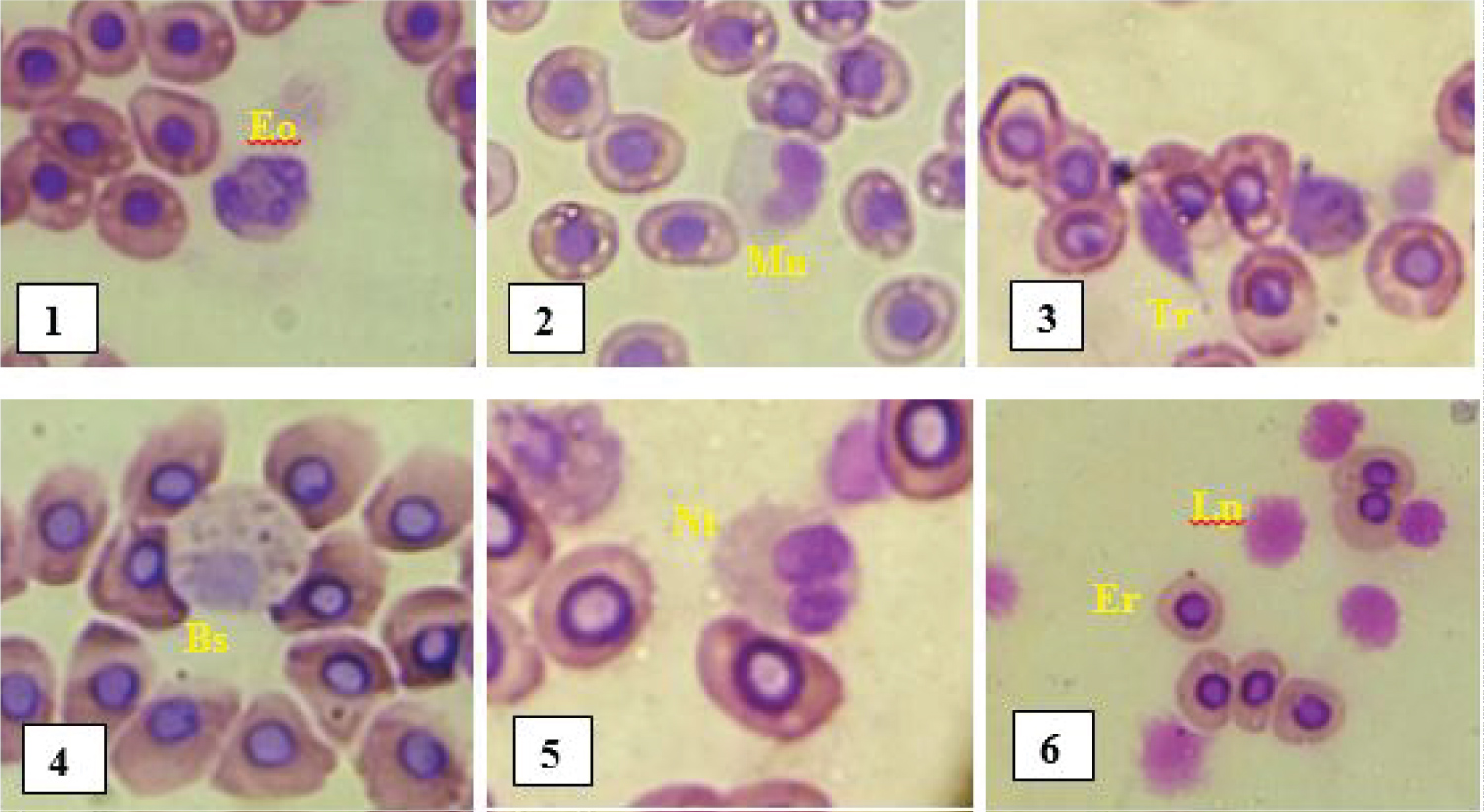 Figure 3: Different blood cells observed in fish.
Figure 3: Different blood cells observed in fish.
Eo: Eosinophil; Mn: Monocyte; Tr: Thrombocyte; Bs: Basophil; Nt: Neutrophil; Er: Erythrocyte; Lp: Lymphocyte.
View Figure 3
The reading of the different slides of the smear made it possible to establish the leukocyte formula of the fish. These are the means ± SD of the different proportions of leukocyte cells contained in the blood of the fish during the exposure (Table 2). The levels of Lymphocytes and Thrombocytes recorded during exposure show no significant difference between the treatments according to sex (p > 0.05). The highest value is noted in T0 (96.88%) in females and in T5 (95.84%) in males. The level of Basophils also shows no significant difference (p > 0.05). But this rate which was 0.20% in control group, gradually increased to reach 0.62% in females T4 and 0.91% in males T5. For the Neutrophil level, the trend is an increased in certain exposed groups and a decreased in other contained groups compared to control. In females, the minimum and maximum values are 1.25% (T3) and 2.58% (T1) against 0.83% (T0). In males, we note respectively 0.84% (T5) and 2.06% (T2) against 1.95% for control. For Eosinophils, there is variation in every direction. The lowest rate is 0.81% (T3) against 1.25% (T0) in females and 0.79% (T5) against 1.18% (T0) in males. Concerning the Monoxytes, the trend is an increased in the level of Monocytes in contaminated groups compared to controls in both males and females.
Table 2: Alterations in differential leukocyte counts in Clarias gariepinus exposed to sub-lethal concentrations of Thalis 112 EC. View Table 2
Total leukocyte count (WBC): Overall, a trend towards an increase in WBC is observed with a significant difference in T2 for males and T5 for both sexes (p = 0.04). The highest values are therefore observed in T5: 236.43*10^3 /μL for females and 237.2*10^3 /μL for males (Figure 4).
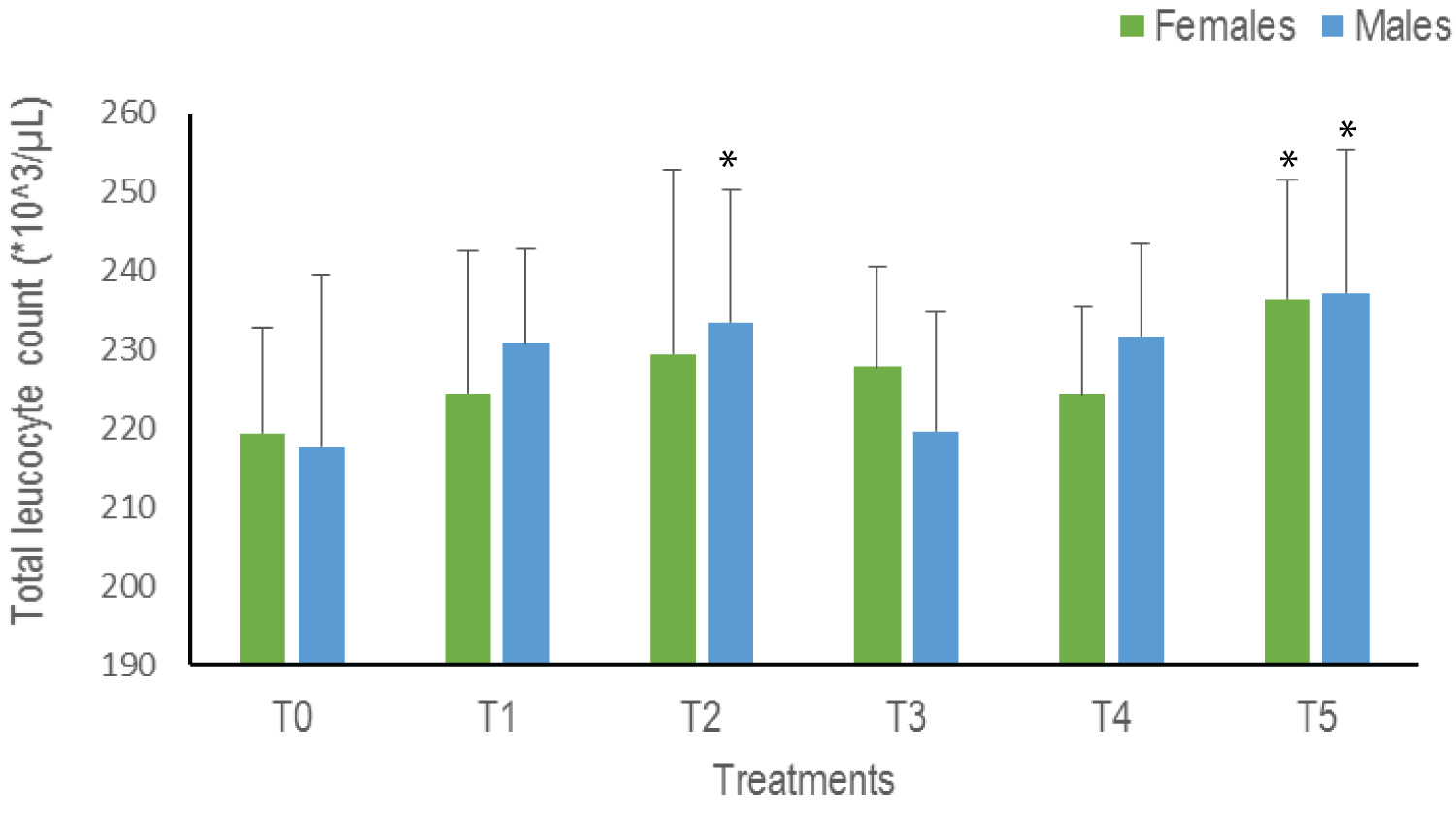 Figure 4: The Total Leukocyte Count of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. For each sex values with asterisk (*) are significantly different with the control T0 at p ˂ 0.05.
View Figure 4
Figure 4: The Total Leukocyte Count of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. For each sex values with asterisk (*) are significantly different with the control T0 at p ˂ 0.05.
View Figure 4
Total erythrocyte count (RBC): Statistical analysis of the RBC data indicates no significant difference when switching from one treatment to another (p > 0.05). However, there is an increasing trend in both sexes with higher rates obtained in T5 (2.78 ± 0.8*10^6 /μL female, 2.93 ± 0.5*10^6 /μL male) whatever the sex (Figure 5).
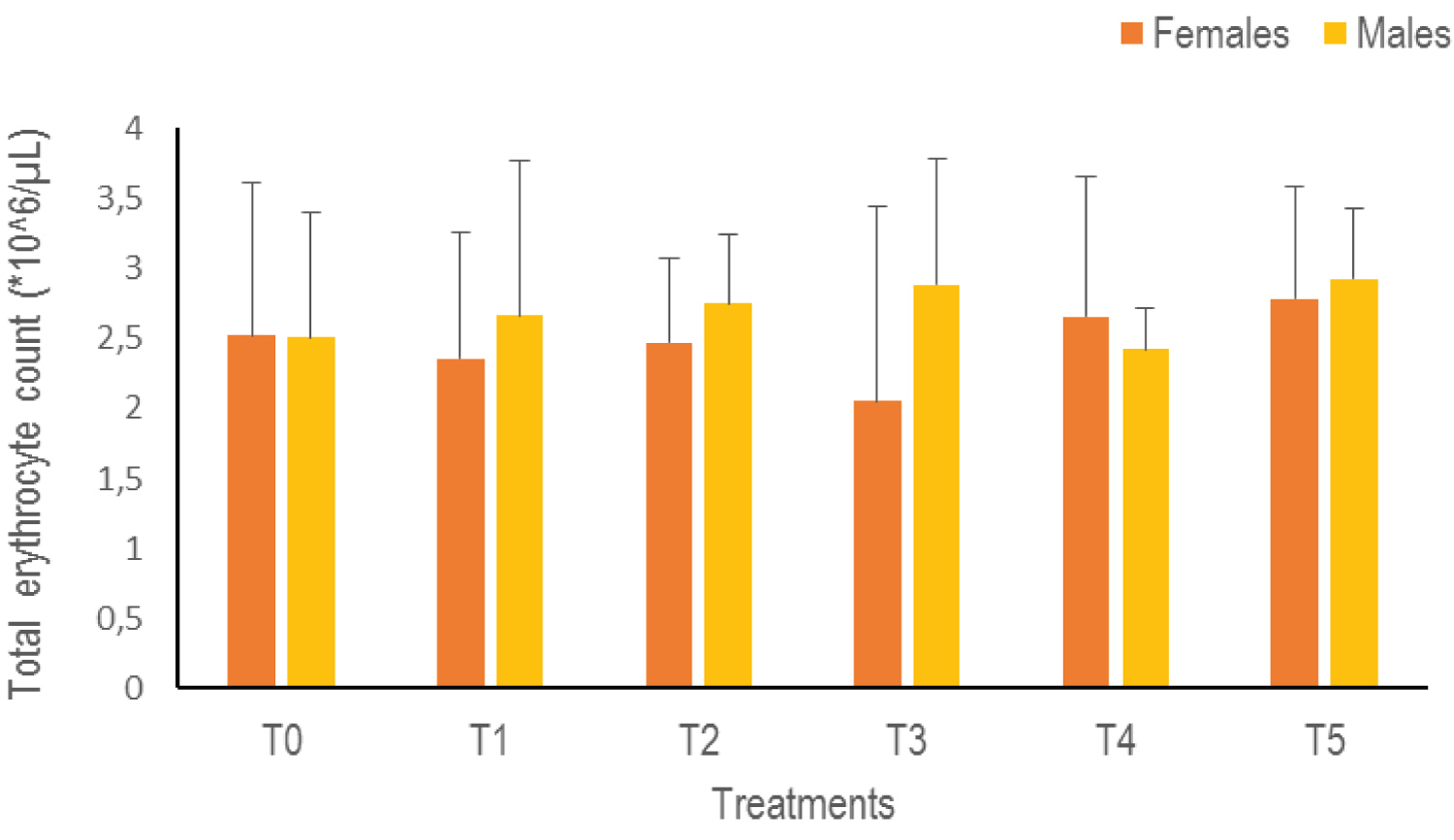 Figure 5: The Total Erythrocyte Count of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 5
Figure 5: The Total Erythrocyte Count of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 5
Haemoglobin content (Hb): The analysis of variances of the Hb does not indicate any statistical difference whatever the sex (p = 0.66). There is nevertheless a progressive increase in the Hb level in the males in all the treatments except in T4. While in females, this value remains more or less constant with a slight increase in T4 (Figure 6).
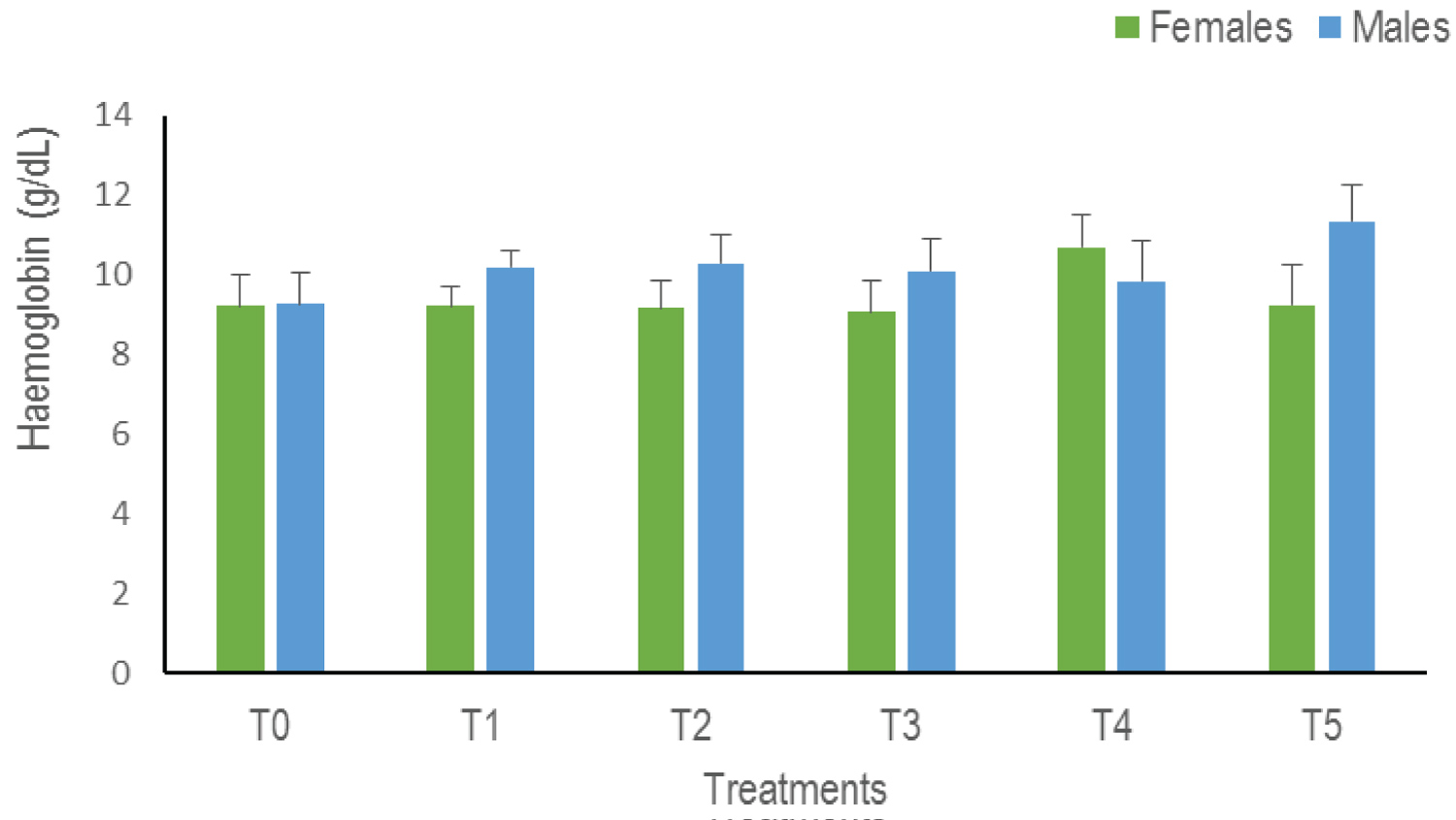 Figure 6: The haemoglobin levels of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 6
Figure 6: The haemoglobin levels of Clarias gariepinus examined after exposed to Thalis 112 ECat a different series of concentration. Data were expressed as mean ± SD. View Figure 6
Erythrocyte constants: Based of the results (Table 3), statistical analyzes of the values of the Erythrocyte constants (MCV, MCH and MCHC)) revealed no significant difference between treatments (p > 0.05). However, a trend of increase was observed in each Erythrocyte constant values.
Table 3: Alterations in Erythrocyte constant values in Clarias gariepinus exposed to sub-lethal concentrations of Thalis 112 EC. View Table 3
The variations of the physico-chemical parameters measured (temperature and pH) during the experiment (before and after renewal of the test solutions) prove to be statistically insignificant. This means that these parameters are identical for all the treatments in this test. The temperature varied from 26.97 to 27.20 °C whatever the period. These values are within the temperature ranges (26 to 30 °C) recommended for optimal growth of C. gariepinus [22,23]. The pH varied from 6.71 to 6.92 whatever the period. These values are in line with the range of 6.5 to 9 recommended for farming C. gariepinus [22,23]. These temperature and pH values are also those obtained by Agbohessi, et al. [24] when the same species was exposed for 13 months to endosulfan and Tihan 175 OTEQ, by Agbohessi, et al. [4,5] during exposure of juvenile C. gariepinus to cotton insecticide Thalis 112 EC and Pyro FTE 472 EC.
Haematological parameters of fish are becoming indispensable tool to assess the impact of pesticides on fish [25]. Thalis exposure resulted into an increase in Total erythrocyte count (RBC) and trend of increase in Haemoglobin(Hb) content and Heamatocrit (PCV) constancy in C. gariepinus along with trend of increase in Mean corpuscular volume (MCV), Mean corpuscular haemoglobin (MCH) and Mean corpuscular haemoglobin concentration (MCHC). As MCH and MCHC are derived from Hb and RBC, increased titre of Hb and RBC would have resulted in the increased MCH and MCHC. Increase in Hb and RBC is suggestive of a strategy used by the fish to increase the ability of oxygen transportation in the blood during periods of metabolic break down [26,27]. According to Singh and Srivastava [28], the RBC elevation may be due to blood cell reserve combined with cell shrinkage as a result of osmotic alterations of blood by the actions of the pesticides. The Erythrocyte constants like MCV, MCH and MCHC seems to be changes which are more sensitive and can cause reversible changes in the homeostatic system of fish. Significant increase in MCH and MCHC has been reported in L. rohita due to exposure to difenoconazole and thiamethoxam [29] and in Barbonymus gonionotus due to quinalphos [30]. Significant increase in MCV was reported in Channa punctatus due to deltamethrin [31] and in B. gonionotus due to quinalphos [30].
Elevated Total leukocyte count (WBC) was observed in this study. Pathophysiological condition of increased WBC is leukocytosis [25]. Reports on WBC reveal a significant increase in its value in C. punctatus under the influence of deltamethrin [31], in C. carpio exposed to fenthion [32], in C. carpio due to monocrotophos [33], in C. punctatus exposed to endosulfan and dimethoate [34], in Heteropneustes fossilis due to chlorpyrifos [35], and in Ctenopharyngodon idella exposed to dichlorvos and its technical grade [36]. WBC formed, either migrateto key organs such as spleen, lymph nodes, gut, or enter the blood. The increased number of leukocytes can occur abnormally as a result of a toxic insult. Sudden increase of WBC may be due to the activation of the animal’s defence mechanism and the immune system. Several chemical compounds including insecticides, generate antibodies due to their interference with immune system which could be the reason for increase in WBC [32]. In the present study the significant increase in WBC may have resulted from the excitation of defence mechanism of the fish to counter the effect of Thalis [37]. The increase in number of leukocytes is a defensive reaction against pesticide stress. These alterations are probably the result of the activation of the immune system in the presence of pesticide, which in turn may be an adaptive response of the fish resulting in a more effective immune defence [38]. It is also this reason which explains the rise in the level of Monoxytes in the present study. This response was also observed in the same C. gariepinus exposed to chlorpyrifos and DDforce [39], and in C. carpio after exposure to phenithrotion and dichlorvos [40,41]. This is also in agreement with earlier studies which have observed similar results after exposure of pesticides such as diazinon [42,43], malathion [44], paraquat [45] and curzate [46]. In the present investigation, a fluctuation in the levels of Neutrophil and Eosinophil with an increase in the levels of Basophils while the whole Lymphocytes + Thrombocytes did not vary, was noted. This differential leukocyte count profile obtained in C. gariepinus is surely linked to the binarity of Thalis consisting of two molecules (Emmamectin benzoate and Acetamiprid) whose modes of action are different and which would have neutralized each other. This effect may also be due to the adjuvant contained in this formulation, which is Thalis.
Immunoglobulin is the most important component of adaptive specific immunity in teleost fish [47]. This parameter shows a significant difference with respect to our results. There is an increase in the fish of the T1 group; which is explained by the fact that the adaptive immune response provided the immune system of fish T1 group with the ability to recognize and remember specific pathogens and then mount a stronger and faster response thanks to memory lymphocytes [47,48]. After a first contact with the toxicant, the fishes of the T1 treatment would have developed a stronger defense. The significant decreases observed in the other treatments would be linked to their high levels of this toxin. Mohammadalikhani, et al. [49] also showed the decrease in IgT in C. idella contaminated with Captan. Decreased immunological activity has also been revealed in several fish due to intoxication with high doses of heavy metals [50].
This investigation clearly demonstrated the adverse impacts of Thalis 112 EC on haematological biomaker of C. gariepinus. Indeed, even if the pollutant has no significant effect on the Heamatocrit level, the Haemoglobin content and the Erythrocyte constants (MCV, MCH and MCHC), it induces an increase in the Total leukocytes count in C. gariepinus. As for Total immunoglobulin, the presence of a low concentration of the pesticide leads to an increase followed by a decrease as the concentration increases. It is concluded that parameter studied in present investigations can be used effectively as potential biomarkers of pesticides toxicity to the fish as well as other aquatic organisms in the field of environmental biomonitoring.
Not applicable.
Not applicable.
All fish experiments were approved by the quality control unit of the university.
Not applicable.
The authors declare that there are no conflicts of interest.
Data generated as part of this study is available upon request from the corresponding author.
All authors have consented to a publication.