Organic halide toxic compounds have penetrated into all natural environments, including natural waters and soil, due to industrial and agro-industrial activity, becoming an obvious danger to human and animal health. One of the factors that can dispose of toxic organic halide compounds is the catalytic activity of the cob(I)alamin cofactor of vitamin B12 through direct or bacterial action. CASSCF geometry optimization of the cob(I)alamin cofactor and each of the 3,5-dibromo-4-hydroxobenzoic acid, 3-bromo-4-hydroxobenzoic acid, and 1,2-dichloroethene common models has been performed. The calculations showed that under the catalytic action of the cob(I)alamin cofactor, the C-Hal. chemical bond from the organic halide compounds breaks heterolytically, releasing a negative bromine or chlorine ion, which binds Van-Der-Waals or chemically to the central cobalt atom of the cob(I)alamin cofactor. The cause of this behavior of the cob(I)alamin cofactor -organic halide compound common models is the formation of intermediate molecular orbitals between the reactants, which are of an antibonding nature as regards the C-Hal. chemical bond and the transfer of electron density from the cob(I)alamin cofactor to an organic halide compound. The mechanisms of organic halide compounds disposal under the action of cob(I)alamin cofactor of vitamin B12 have been drowned. Thus, vitamin B12 can be applied as an antitoxic remedy in organohalide poisoning (Graphical Abstract).
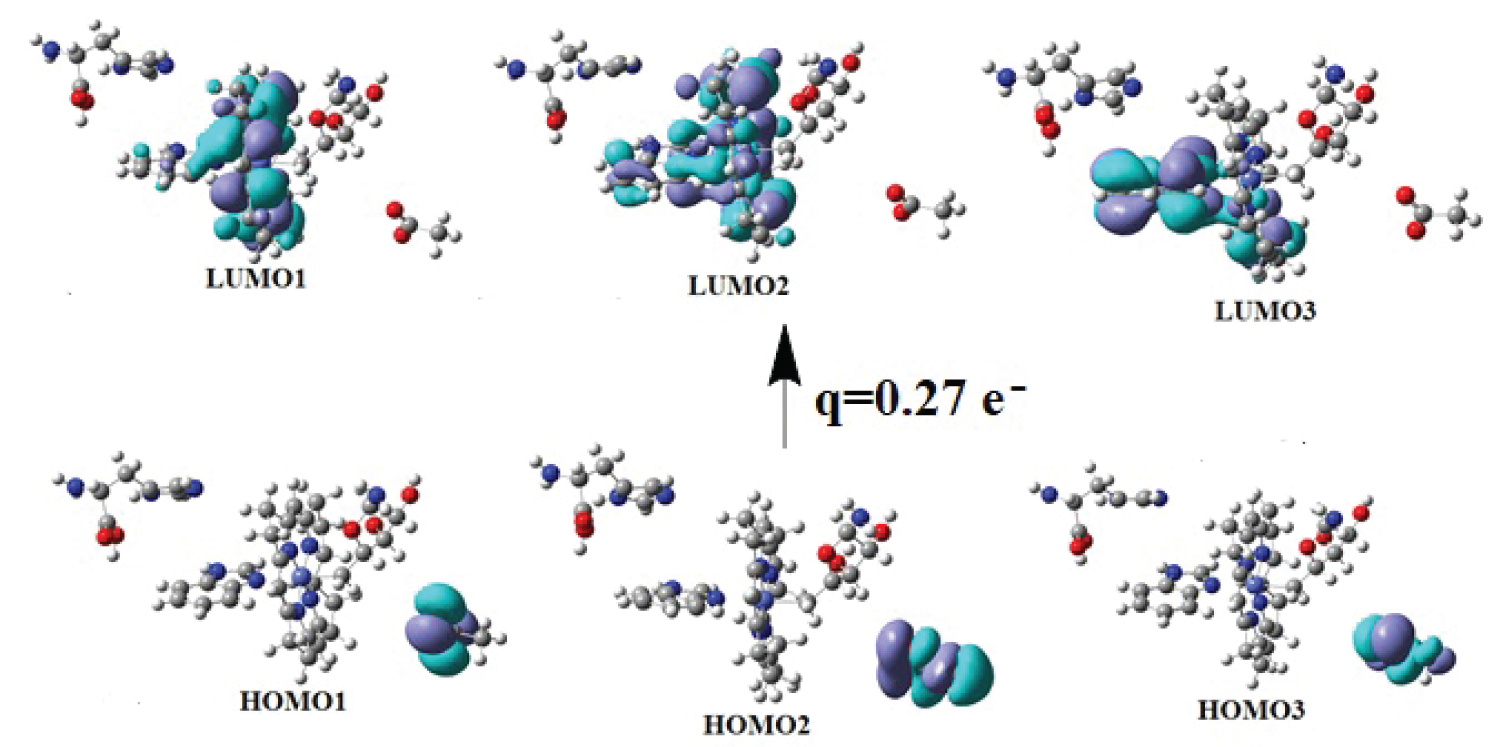 Graphical Abstract.
View Graphical Abstract
Graphical Abstract.
View Graphical Abstract
Organic halide compounds, MCSCF, Vitamin B12, Cob(I)alamin, Environmental chemistry
Organic halide compounds have been used very intensively in industry and agriculture as solvents, plastic agents, compounds for the preparation of medicines, and dyes, but also as agents against pests of agricultural crops. Over several decades, these compounds have accumulated enormous amounts in all natural environments, especially in soils, water basins, and underground waters [1-6]. Given that these compounds are dangerous for human and animal health, their elimination from the environment has become a pressing problem in most countries of the world, including the US [5]. Organochlorine compounds (OCCs), which are the most used part of the organic halide ones, contain at least one covalently bound chlorine atom, having a wide variety of structures with very diverse chemical properties. Tests on various animal species including fish, frogs, rats, bats and humans have shown positive results for organochlorine pesticide toxicity. A large population declines have been observed in various animal species due to the toxicity of OCCs. The individual organohalide compounds that are part of persistent organic pollutants have diverse toxicological properties that confer adverse biological effects on fish, wildlife and humans [7].
The environment's state influences are enormous if we consider that organic halides are very stable in the environment and actually can be presented as carcinogenic agents [6]. Experimental data have shown that removing halogens from organic halide compounds is possible by using reducing agents, although in some cases, the dehalogenation is incomplete [1-3,6,8-13]. The organic halide compounds’ reductive dehalogenation has been studied using the DFT method [14]. The calculations demonstrated that the orbitals of the halogen bonds do not participate in the highest occupied molecular orbitals (HOMO); instead, they participate with a maximum contribution to the lowest unoccupied molecular orbitals (LUMO). This explains the breaking of halogen bonds in organic halide compounds under the influence of reducing agents. Also, theoretical calculations showed that the elimination of halogens from organic halide compounds occurs sequentially.
Considering the possibility of worsening the ecological situation and the protection of both the biosphere and the population’s health due to the penetration of organic halide compounds, the degradation of these compounds is becoming an increasingly pressing problem. One of the agents that can be used to detoxify these organic compounds is vitamin B12. The dehalogenation of these organic halide compounds can occur through the direct use of vitamin B12 [15] or through bacterial activity in the mechanism of which the participation of vitamin B12 or similar compounds is well known [16-19]. Anaerobic reductive bacteria can be a solution for the dehalogenation of organic halide compounds in natural environments. They detoxify both organic halide compounds of aliphatic and aromatic nature of various structures [20-22]. This suggests that the reductive mechanism of dehalogenation of organic halide compounds may be similar for all organic halide compounds. Organohalide -respiring bac1eria, for example, transfer an electron to organic halide compounds, using their decomposition energy for growth [23-27]. It has demonstrated that Dehalococcoides use vitamin B12 cofactor cob(I)alamin in the reductive detoxification of organic halide compounds [23-27].
The interaction between cob(I)alamin and the organic halide compounds was supposed to occur through the halogen atoms and the central cobalt atom [17,18]. The DFT method was used to study the nature of this interaction in Dehalococcoides mccartyi strain CBDB1 [17,27,28]. Zhang, et al. have developed a donor-acceptor theory between the cob(I)alamin cofactor and organic halide compounds through which they imitate the transfer of electron or electron density in the process from the highest occupied molecular orbital of the vitamin B12 cofactor to the lowest unoccupied molecular orbital of the organic halides through the cobalt and halogen atoms of the compounds [17,18].
The methylcobalamin and adenosylcobalamin cofactors are the basic structures for the catalytic action of vitamin B12. In the center of these structures is the cobalt ion, which is linked by four coordinative bonds to the coring ring (Figure 1), which structure is planar [29]. The permanent axial ligand to the central cobalt atom in the relaxed state ( in vitro ) is dimethylbenzimidazole, which is linked to the corrin ring through a side chain. In the in vivo catalytic action, this ligand is usually replaced by a histidine molecule. In the position of the other axial ligand is one of the methyl or 5'-deoxy-5'-adenosyl radicals. In their biochemical processes, the central cobalt atom of the cofactors changes its oxidation state from +3 to +1, and vice versa [29] (Figure 1). As it has been shown, the effective particle in the process of annihilation of toxic organic halide compounds is the cofactor of vitamin B12 in which the oxidation state of the central cobalt atom is +1, and the axial ligands are decoupled from the central atom [14].
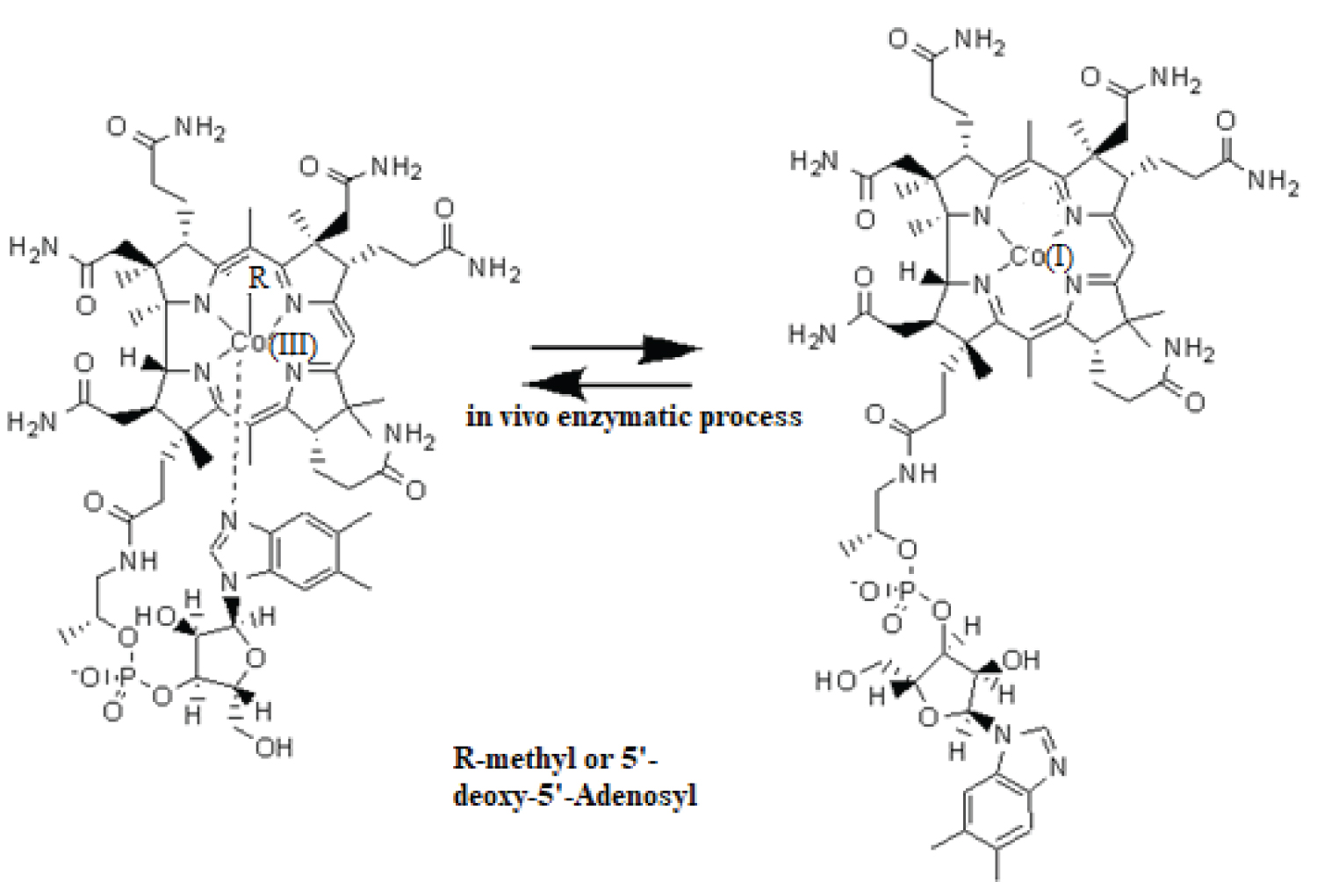 Figure 1: The schematic picture of the in vivo vitamin B12 cofactors activity with the central atom converting from Co(III) to Co(I) and vs. versa. Left is a Co(III) structure; Right is a Co(I) structure.
View Figure 1
Figure 1: The schematic picture of the in vivo vitamin B12 cofactors activity with the central atom converting from Co(III) to Co(I) and vs. versa. Left is a Co(III) structure; Right is a Co(I) structure.
View Figure 1
It should be mentioned that despite the remarkable efforts of the research community, both experimentally and theoretically, the real mechanism of halogen bonds breaking reaction in organic halide compounds under the influence of cob(I)alamin cofactor of vitamin B12 is not known at the electronic level. Unfortunately, the modeling of the orbitals mixing, e. g. of the Pseudo-Jahn-Teller effect, and, respectively, the transfer of the electronic density from the highest occupied molecular orbitals of one reagent to the lowest unoccupied molecular orbitals of another reagent in their common model are out of the limits of the DFT method, as was proved by Bersuker [30]. For these purposes, it is necessary to use the multi-configurational self-consistent (MCSCF) method, which is the task of this paper.
Geometry optimization of cob(I)alamin cofactor structure and organic halide compounds common models have been performed using the CASSCF method. 13 electrons and 13 orbitals were used for the active zone of these electronic structure calculations. We aimed for the molecular orbitals most responsible for the interaction between the reactants through which the transfer of electron densities from the highest occupied molecular orbitals to the lowest unoccupied molecular orbitals takes place within the limit of mixed orbitals and the Pseudo-Jahn-Teller-Effect. According to electronic structure calculations with the participation of vitamin B12 cofactors, this approximation is enough to achieve the proposed goal [31-33]. According to our estimates, the increase in the number of electrons and orbitals in the active zone of the CASSCF method did not lead to observable changes in the calculation results. On the other hand, modifying the interaction between the reactants by increasing the number of electrons and orbitals would only increase the effects of orbital mixing and the Jahn-Teller-Pseudo-Effect, which are responsible for the studied reactions. The 6-311G ** basis set was used for bromine atoms, the 6-31G ** basis set was used for Co and Cl atoms, and the 6-31G basis set was used for the rest of the atoms included in electronic structure calculations. The NwChem code was used for all geometry optimization performed in this paper [34]. The semi-newton optimization procedure with line searches and approximate energy Hessian updates embodied in the NwChem software has been used as the self-consistent procedure. In general, it is considered to add in the CASSCF calculations the dynamic contributions to the correlation energy in order to obtain a complete procedure in the CASPT2 method. But, given that results agree with the experimental data, our calculations correctly reflect the studied processes.
As the experiment shows, the cob(I)alamin cofactor catalyzes the detoxification of both aliphatic and aromatic organic halide compounds [20-22]. The interaction between the cob(I)alamin cofactor and the organic halide compounds can take place in various geometric forms of the interaction between the reagents, the most probable being the approach of the organic compound in a quasi-perpendicular or quasi-parallel manner to the corrin ring of the cob(I)alamin cofactor. In the first case, the organic halide compound can directly interact with the central cobalt atom through a halogen atom. In the second case, the organic halide compound can interact with the molecule of the cob(I)alamin cofactor through π-π bonds of a corrin ring of the cob(I)alamin cofactor and of the aromatic or unsaturated organic halide compound. It is interesting that the experimental data point to the interaction model between the reactants, in which the organic halide compound is quasi-perpendicular to the corrin ring of the cob(I)alamin cofactor so that the reactants interact through the central cobalt atom of the first with a halogen atom of the second reactant [17-18]. In particular, the dehalogenation of 3,5-dibromo-4-hydroxobenzoic acid, 3-bromo-4-hydroxobenzoic acid [19], and 1,2-dichloroethene organic halide compounds have been reported [15]. These organic halide compounds were selected to create common computational models for CASSCF geometry optimizations (Figure 2). For convenience, the side chains of the cob(I)alamin cofactor of vitamin B12 were substituted by hydrogens. The side chain, which connects the dimethylbenzimidazole axial ligand with the corrin ring, contains a negative charge through the acid residue =HPO 4 - , so we modeled a negative charge through an OH - ion, which has been placed at a distance greater than 10 Å from the rest of the model to have a total charge of the calculated models balanced with the actual total charge of the calculated models. At the start of the CASSCF geometry optimization procedures, the reactants were located in the geometric relationship of the quasi-perpendicular position of the organic halide compounds on the corrin ring surface of the cob(I)alamin cofactor. Besides this, the reactants were located at a distance of at least 4.00 Å from each other, at which there is generally accepted no chemical bonding between the reactants.
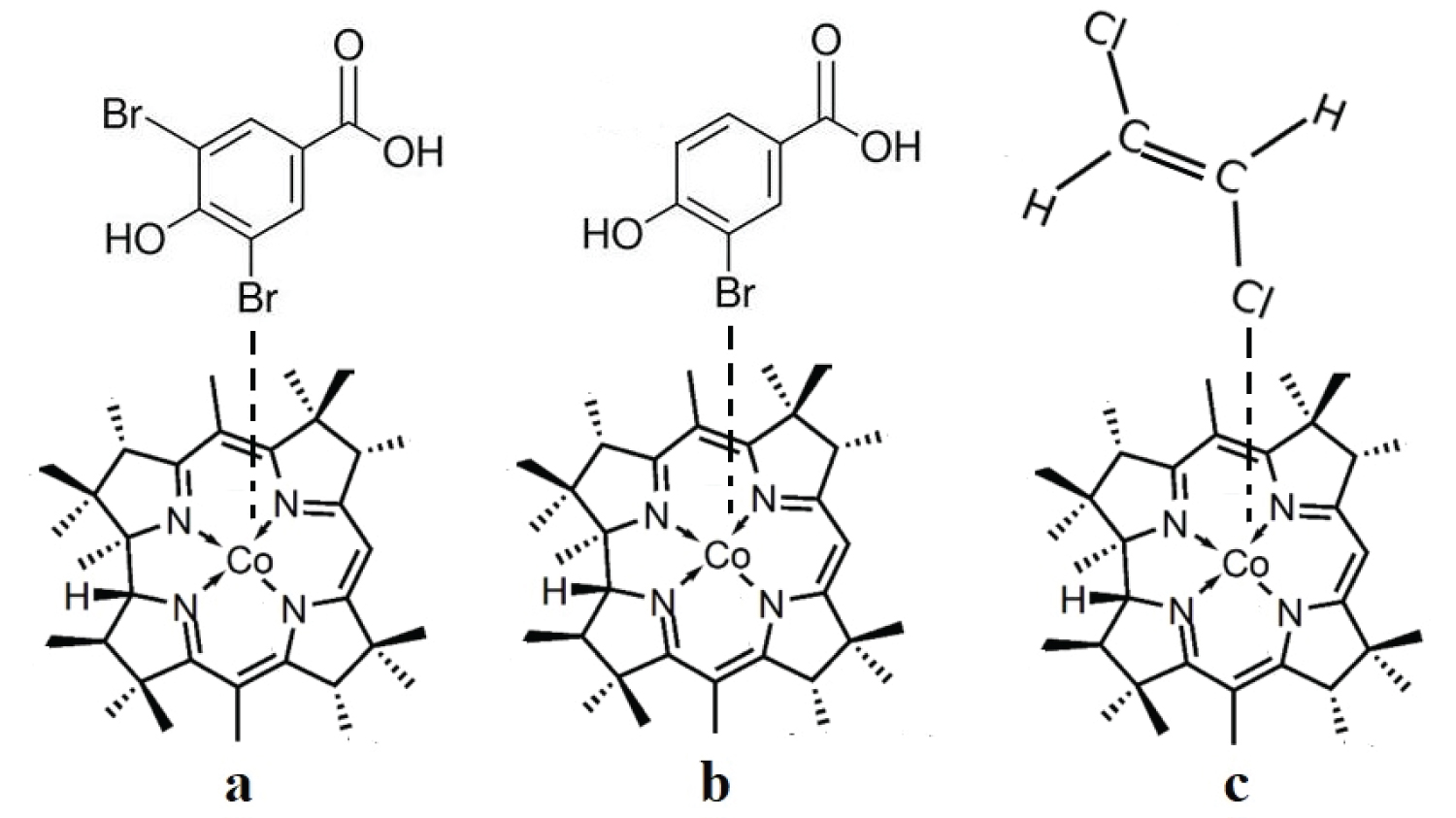 Figure 2: The schematic picture of the common models' cob(I)alamin cofactor -organic halides used in the CASSCF geometry optimization procedures: a) cob(I)alamin cofactor and 3,5-dibrom-4-hidroxobenzoic acid; b) cob(I) alamin cofactor and 3-bromo-4-hidroxobenzoic acid; c) cob(I)alamin cofactor and 1,2-dichloroethene.
View Figure 2
Figure 2: The schematic picture of the common models' cob(I)alamin cofactor -organic halides used in the CASSCF geometry optimization procedures: a) cob(I)alamin cofactor and 3,5-dibrom-4-hidroxobenzoic acid; b) cob(I) alamin cofactor and 3-bromo-4-hidroxobenzoic acid; c) cob(I)alamin cofactor and 1,2-dichloroethene.
View Figure 2
At the start of the CASSCF geometry optimization procedures, several intermolecular molecular orbitals were created in the active area of the CASSCF electronic structure calculations, which were kept throughout the whole geometry optimization processes. Two of them in the cases of the cob(I)alamin cofactor -3,5-dibromo-4-hydroxobenzoic acid and cob(I)alamin cofactor -3-bromo-4-hydroxobenzoic acid models are populated with approximately 2 and 1 electrons, respectively, and an intermolecular molecular orbital is populated with approximately 2 electrons and each of two others are populated with approximately 1 electron in the case of the cob(I)alamin cofactor -1,2-dichloroethene model (Figure 3). The other intermolecular molecular orbitals in the CASSCF active area of the electronic structure calculations of the calculated models have a population about equal to ten times smaller or less. We can consider their influence on the chemical processes less important.
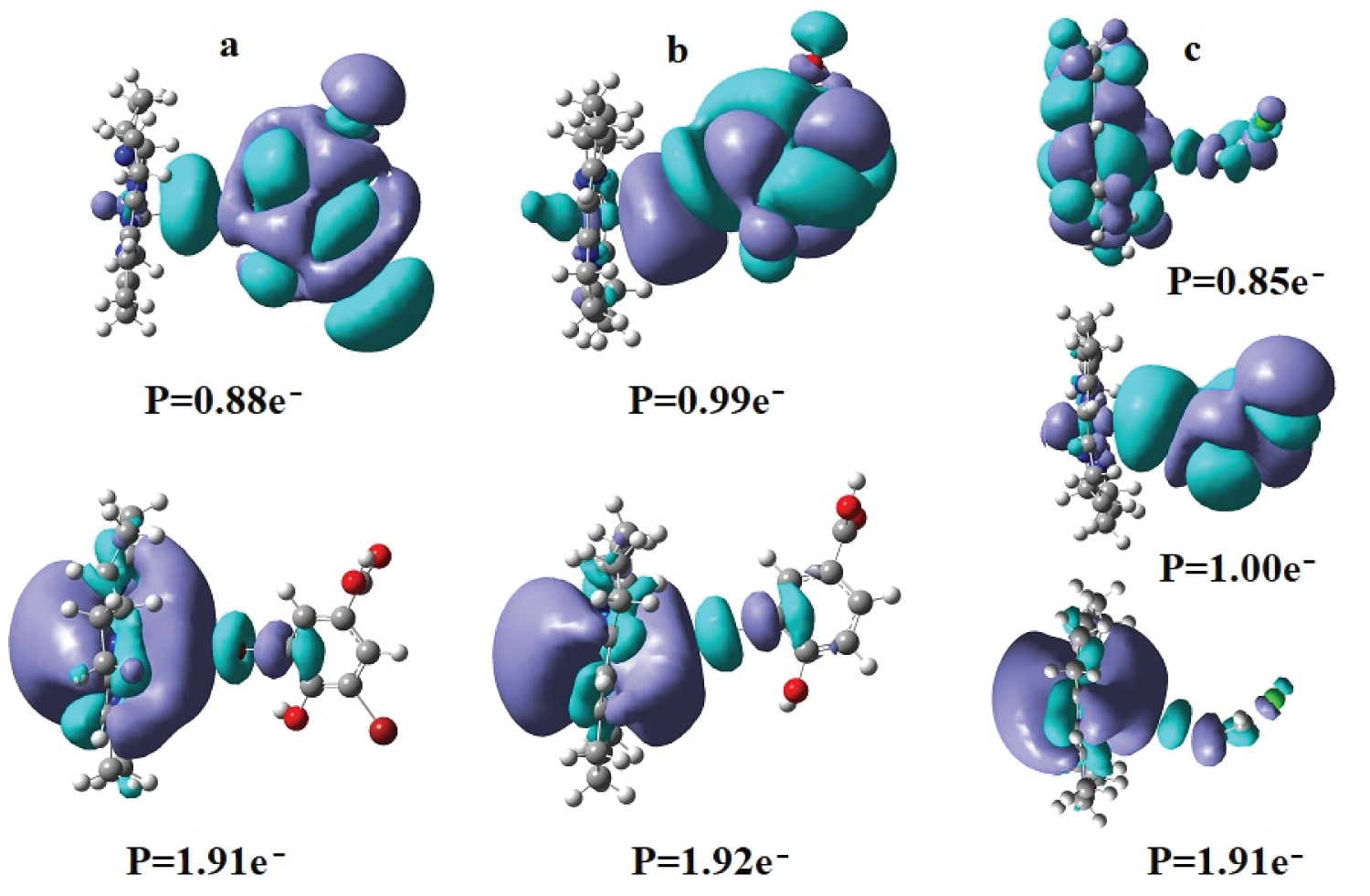 Figure 3: The surfaces of the intermolecular molecular orbitals and their electron density populations (P) in the cob(I)alamin cofactor-organic halide compound models: a) cob(I)alamin cofactor and 3,5-dibromo-4-hidroxobenzoic acid; b) cob(I)alamin cofactor and 3-bromo-4-hidroxobenzoic acid; c) cob(I)alamin cofactor and 1,2-dichloroethene.
View Figure 3
Figure 3: The surfaces of the intermolecular molecular orbitals and their electron density populations (P) in the cob(I)alamin cofactor-organic halide compound models: a) cob(I)alamin cofactor and 3,5-dibromo-4-hidroxobenzoic acid; b) cob(I)alamin cofactor and 3-bromo-4-hidroxobenzoic acid; c) cob(I)alamin cofactor and 1,2-dichloroethene.
View Figure 3
Two factors that influence the nature of the interaction between the cob(I)alamin cofactor and organic halide compounds should be mentioned. First of all, the intermolecular molecular orbitals are the result of the interaction of the highest occupied molecular orbitals of the cofactor cob(I)alamin (calculated in the approximation of a mono-determinant method) [19] with the lowest unoccupied molecular orbitals of the organic halides compounds. In this sense, the intermolecular molecular orbitals show a transfer of electron density from the cob(I)alamin cofactor to the organic halide compounds. It should be noted that the electron density transfer from the cob(I)alamin cofactor to organic halide compounds is not understood in the frame of classical chemistry. This occurs only if the electronic density is transferred from one reagent's isolated orbitals to another reagent’s isolated orbitals. In our case, the formation of intermolecular molecular orbitals takes place from the HOMO of the cob(I)alamin cofactor (which are occupied with two electrons in a mono-determinant calculation) [19] and LUMO (which are not occupied at all with electrons in the mono-determinant calculation) [19] of organic halide compounds. The formation of common intermolecular orbitals between these two types of orbitals, which are massively populated with electron densities, leads to the population of the orbitals placed on both reagents with electron densities, that is, formally leads to a transfer of electron density from the HOMO of the cob(I)alamin cofactor to LUMO of organic halide compounds. This kind of electron density transfer is valid only if the interaction between the two reagents occurs when these common intermolecular molecular orbitals exist. Secondly, the intermolecular molecular orbitals are of an antibonding nature as regards the C-Hal. the chemical bond in organic halide compounds and their massive population with electron densities stimulate the weakening or complete breaking of these chemical bonds during the interactions of cob(I)alamin cofactor - organic halide compounds.
The CASSCF geometry optimization procedures led, in the case of all three models, to the breaking of the C-Hal. bonds. Interestingly, C-Hal. bond breaking is heterolytic, producing a negative bromine or chlorine ion. The curious thing is that throughout the CASSCF geometry optimization procedures, the bromine or chlorine ion remains linked by a Van-Der Waals bond to the central cobalt atom at a distance slightly less than 4.00 Å while the rest of the organic halide molecule moves away from the halogen ion at a distance greater than 4.00 Å, showing that the C-Hal. bond in organic halide compounds breaks completely under the catalytic influence of the cob(I)alamin cofactor. These results are in complete agreement with the experimental data, which show that under the catalytic influence of the cofactor cob(I)alamin, the C-Hal. chemical bonds in organic halide compounds are broken, and the halogen ions resulting from this catalytic process remain interacting with the central cobalt atom [16,19]. It should be mentioned that at this stage, the bromine or chlorine ions do not bond chemically and completely with the central cobalt atom, instead form a Van-Der-Waals bond because the cob(I)alamin cofactor is a tetra-coordinating structure with its ligands as long as the oxidation state of the cobalt ion remains equal to +1. When the oxidation state of the central cobalt atom changes from +1 to +2 or +3 (the modifications, which are commonly accepted), by giving up electrons to other substrates, the bromine or chlorine ion will form a chemical bond with the central cobalt atom, since the vitamin B12 cofactors are penta- or six-coordinate in these cases. The mechanisms of the catalytic influence of the cob(I)alamin vitamin B12 cofactor on the organic halide compounds are shown in Figure 4, Figure 5 and Figure 6.
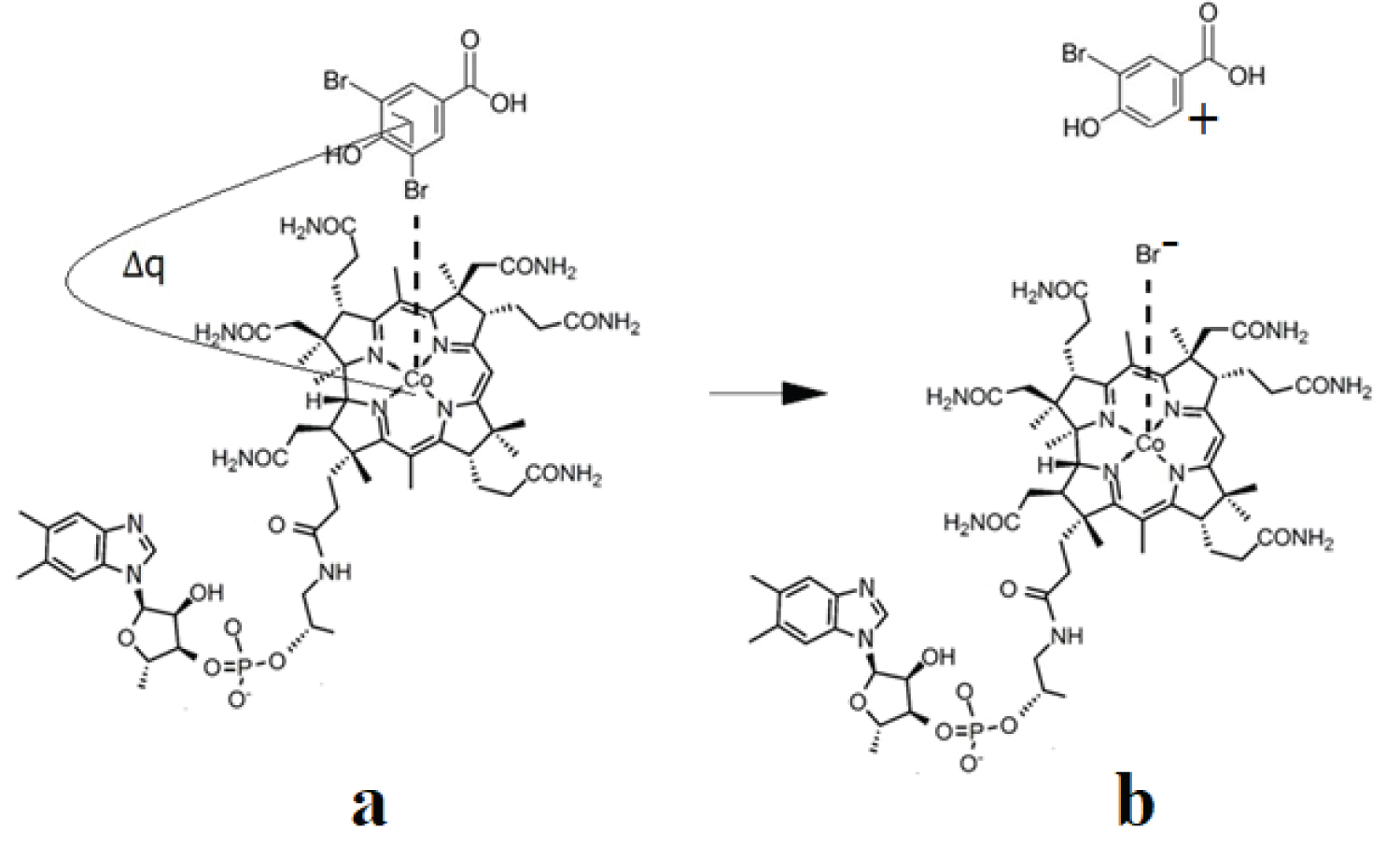 Figure 4: The schematic mechanism of the cob(I)alamin cofactor interaction with 3,5-dibromo-4-hydroxybenzoic acid: a) Initial reagents; b) Products.
View Figure 4
Figure 4: The schematic mechanism of the cob(I)alamin cofactor interaction with 3,5-dibromo-4-hydroxybenzoic acid: a) Initial reagents; b) Products.
View Figure 4
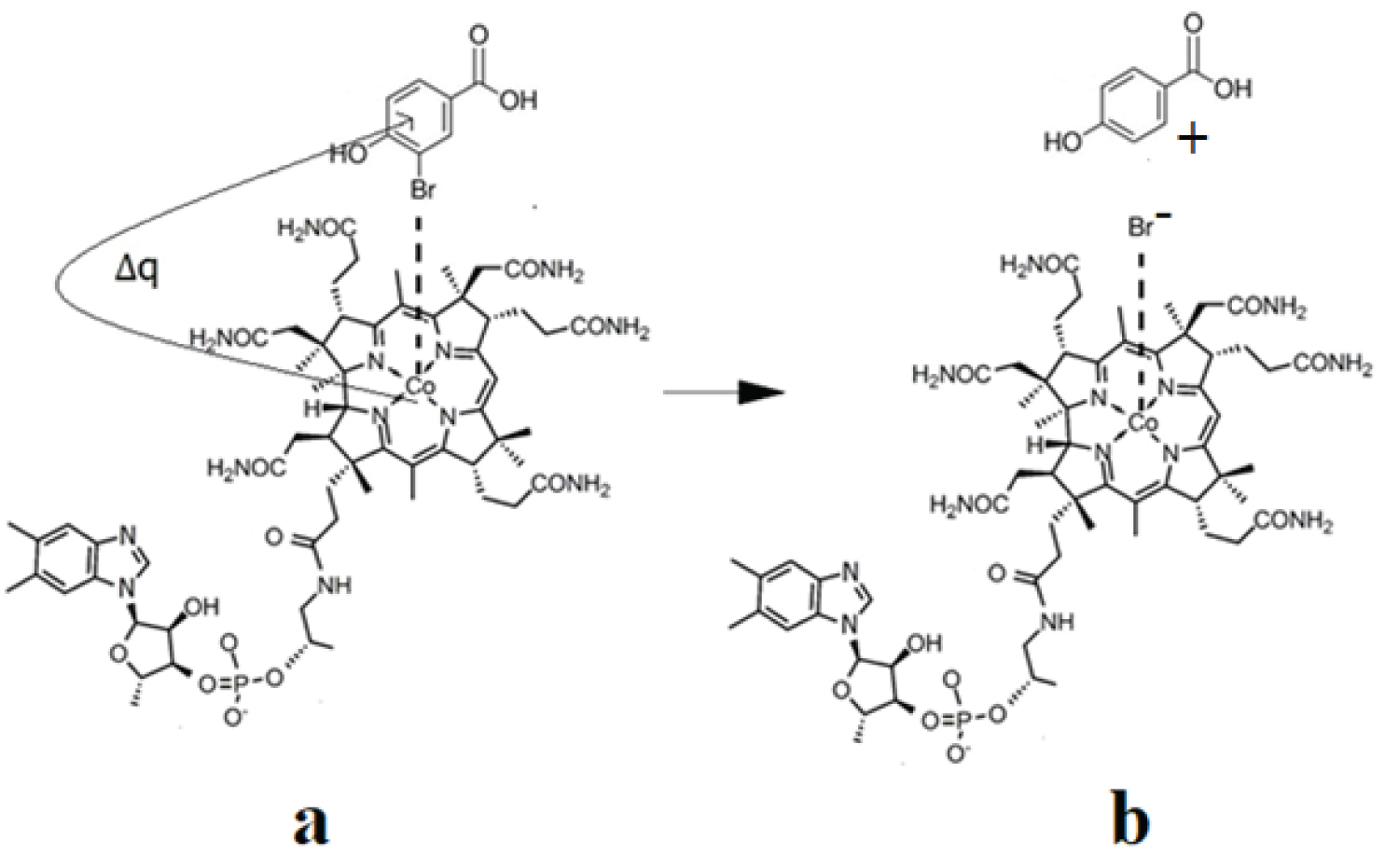 Figure 5: The schematic mechanism of the cob(I)alamin cofactor interaction with 3-bromo-4-hydroxybenzoic acid: a) Initial reagents; b) Products.
View Figure 5
Figure 5: The schematic mechanism of the cob(I)alamin cofactor interaction with 3-bromo-4-hydroxybenzoic acid: a) Initial reagents; b) Products.
View Figure 5
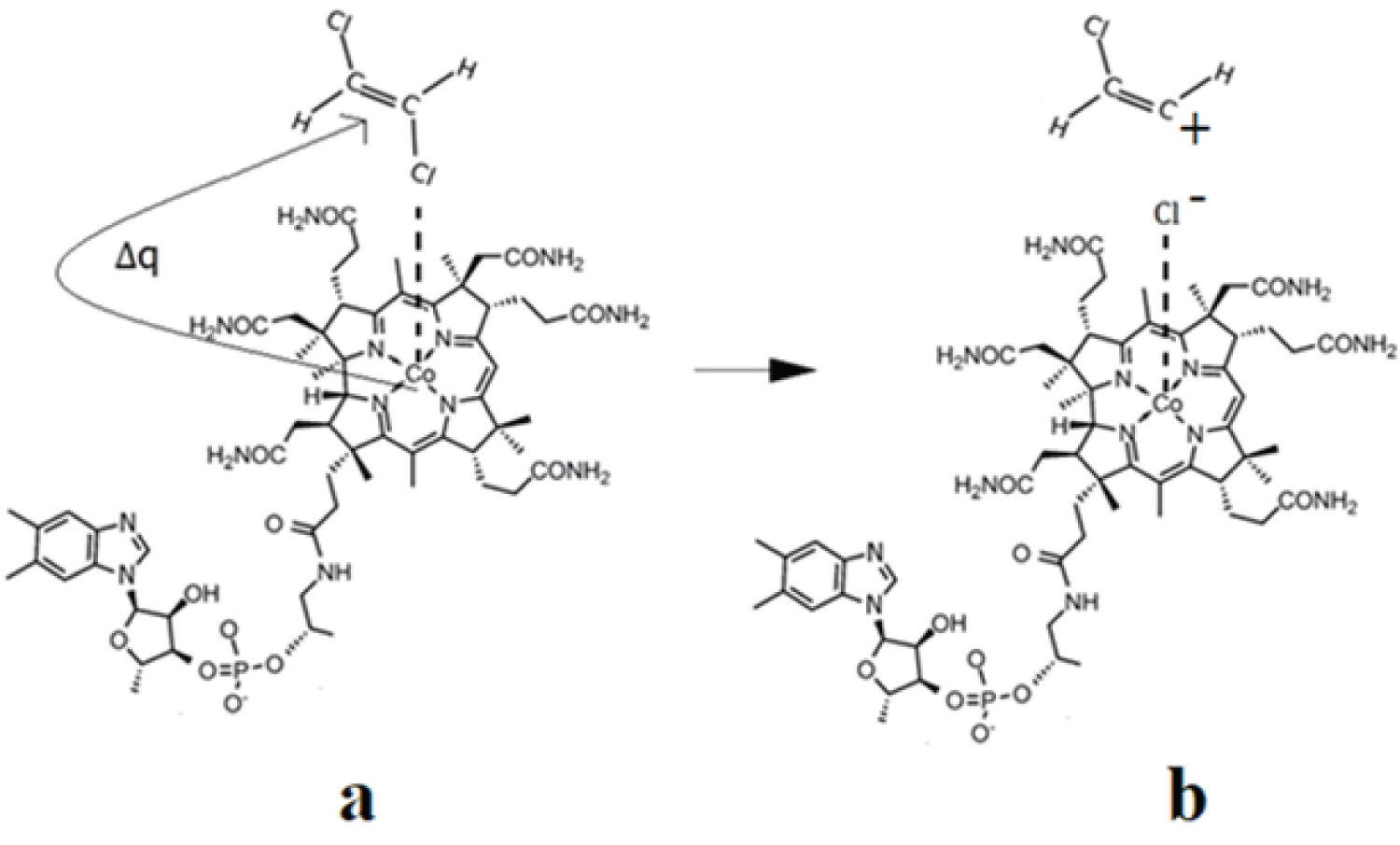 Figure 6: The schematic mechanism of the cob(I)alamin cofactor interaction with 1,2-dichloroethene: a) Initial reagents; b) Products.
View Figure 6
Figure 6: The schematic mechanism of the cob(I)alamin cofactor interaction with 1,2-dichloroethene: a) Initial reagents; b) Products.
View Figure 6
To ensure the correctness of the calculated models, we performed the CASSCF geometry optimization procedures of the models in which the organic halide compounds act on the cob(I)alamin cofactor being placed in parallel or oblique geometry to its corrin ring plane. The results of the calculations show that in these models, the catalytic interaction between the reagents is lower and produces bromine or chlorine ions by breaking the C-Hal. bond of the organic halide compounds only if there is a direct π-π interaction between the cob(I)balamin cofactor corrin ring and the molecular plane of aromatic organic halide compounds. Unfortunately, this was possible only if the distance between the reactants was less than 4.00 Å. Considering that the real structure of the cob(I)alamin cofactor includes chain sides, which prevents the too-close proximity between the organic halide compounds and the corrin ring of the cob(I)alamin cofactor, these reactions are less possible. Therefore, the most probable catalytic interaction between the organic halide compounds and the cob(I)alamin cofactor, which leads to the disposal of organic halides, is through the halogen and cobalt atoms in the quasi-perpendicular position of the organic halide compound on the corrin ring of the cob(I) cofactor of the vitamin B12.
The CASSCF geometry optimization procedures show that the cob(I)alamin cofactor of vitamin B12 acts catalytically on organic halide compounds, 3,5-dibromo-4-hydroxobenzoic acid, 3-bromo-4-hydroxobenzoic acid, and 1,2-dichloroethene forming with each of them several common intermolecular molecular orbitals. Two of these, in the case of the first two models, are populated with approximately two and one electrons, respectively, and one of them is also populated with approximately two electrons, the other two being populated with approximately one electron in the case of the last calculated model. These intermolecular molecular orbitals are of an antibonding nature to the C-Hal. bond of organic halide compounds. As a result of these interactions between the cob(I)alamin cofactor of vitamin B12 and the organic halide compounds, the electron density is transferred from the cob(I)alamin cofactor to each of the organic halide compounds. Due to these factors, the C-Hal. bond in the organic halide compounds breaks heterolytically, producing a negative halogen ion, which, in turn, binds to the central cobalt ion of the cob(I)alamin cofactor through a Van-Der-Waals bond and the remainder of the organic halide compound. Following the change in the oxidation state of the cobalt ion from +1 to +2 or +3, the Co-Hal. Van-Der-Waals bond turns into a chemical bond. The most probable geometric relationship between the reactants is the one in which the organic halide compound interacts with the cob(I)alamin cofactor through a halogen atom of the organic halide compound with the central cobalt atom of the vitamin B12 cob(I)alamin cofactor being almost or totally perpendicular to its corrin ring plane. The dehalogenation of the organic halide compounds under the catalytic influence of the cob(I)alamin cofactor is sequential. In conclusion, vitamin B12, which is a regular part of modern medicine nervous diseases treatment, is also an antitoxic remedy in organohalide poisoning (Supplementary information).
This work was carried out within the Moldovan State Program (2020-2023) “Study and management of pollution sources to develop recommendations for implementing measures to mitigate the negative impact on environment and human health”, Project cipher: 20.80009.7007.20. This research was supported, in part, under National Science Foundation Grants CNS-0958379 and CNS0855217 and the City University of New York High-Performance Computing Center at the College of Staten Island and by the National Science Foundation through TeraGrid resources provided by the TeraGrid Science Gateways program under grants CHE090082 and CHE140071.