Background: Historically with pancreatic trauma, complete disruption of the main pancreatic duct (MPD), classified as Grade IV-V by the American Association for the Surgery of Trauma (AAST), necessitated a damage-control laparotomy. This was to avoid mortality, shorten diet upgrade timeframe, and hence shorter length of stay. However, acute pancreatic resection entailed complications of pancreatic fistulas and leaks.
With the advance of imaging-guided interventions, non-operative management such as percutaneous and transpapillary drainage of traumatic peripancreatic collections have been trialled favourably. The aim of this case series is to evaluate the efficacy of endoscopic ultrasound-guided (EUS) transmural drainage in managing traumatic peripancreatic collections as a less invasive alternative to traditional approaches. This study also highlights the importance of anatomical knowledge regarding peripancreatic collection's common location in the lesser sac, the pancreas relationship to adjacent organs, and the formation of the main pancreatic duct in regards to the feasibility of therapeutic internal drainage.
Methodology: A retrospective case series was conducted at a single tertiary endoscopy unit, analysing patient data over a 5-year period. Inclusion criteria outlined patient's age 5 to 80-years-old, traumatic pancreatic injury of at least Grade IV and haemodynamic stability. Exclusion criteria involved previous episodes of pancreatitis or abdominal trauma. Patient demographics and clinicopathological characteristics were retrospectively collected.
Results: The study identified 7 patients with traumatic pancreatic injuries that were managed from 2018-2022; age ranging from 5 to 34-years-old, with majority being female (n = 5). Majority of the mechanisms of trauma were a handlebar injury (n = 4). Diagnosis was confirmed with an elevated lipase and computerized tomotography (CT) confirmation of proximal pancreatic transection with MPD disruption. All patients sustained an isolated single organ grade IV pancreatic injury, except case 4 and 5 with other intra-abdominal visceral Grade 1 injuries.
6 patients underwent early ERCP-guided transpapillary drainage with 1 being unsuccessful for pancreatic duct stent insertion (case 1) and 1 complication of stent migration (case 2). Surveillance imaging post ERCP showed the stents were unable to bridge the disrupted duct and development of symptomatic collections with an average size of 9.9 cm. Hence, all patients proceeded to EUS-guided transmural drainage; with 2/7 patients requiring repeat drainages (case 6 and 7).
Majority (n = 6) had a cystogastrostomy, whilst 1 (case 6) had a cystoenterostomy due to feasibility of the peripancreatic collection being adjacent to duodenum rather than stomach. However, case 6 subsequently required repeat EUS-guided drainage with cystogastrostomy for ongoing collections. Hence all patients avoided initial laparotomy with an average index length of stay of 11.7 days. Successful transmural drainage was demonstrated, with no long-term complications of pancreatic insufficiency; except for 1 patient requiring a distal pancreatectomy at 2 year follow-up due to chronic pain.
Conclusion: The early results of this series support EUS-guided transmural drainage as a viable management option for traumatic peripancreatic collections, showcasing successful outcomes, minimal complications, and long-term efficacy in avoiding surgical interventions. More studies are required before the adoption of this procedure as a less invasive and complication-prone management approach for traumatic peripancreatic collections.
Endoscopic ultrasound, Cystogastrostomy, Pancreatic trauma, Traumatic peripancreatic collection, Transmural drainage
Compared to other blunt abdominal traumas, the incidence of pancreatic trauma is low [1]. Nevertheless, it poses significant morbidity due to a wide range of complications such as pancreatitis, pseudocysts, strictures and endocrine/exocrine insufficiency. Historically, complete disruption of the main pancreatic duct (MPD), classified as Grade IV-V by the American Association for the Surgery of Trauma (AAST), necessitated a damage control laparotomy [2].
With the advance of imaging-guided intervention, non-operative management has been trialled with favourable outcomes [3-6]. We present a case series of 6 paediatric and 1 adult patient with traumatic MPD disruption and subsequent novel management of peripancreatic collections with endoscopic ultrasound-guided (EUS) transmural drainage. Performed at a single tertiary institution, we will analyse the procedural success and complication rate. We will also discuss current options such as percutaneous drainage and endoscopic retrograde cholangiopancreaticography (ERCP) transpapillary drainage [7-10].
Our inclusion criteria were patient’s age 5 to 80 years, with a traumatic pancreatic injury of at least Grade IV with MPD disruption and subsequent peripancreatic collection. Patients had to have no previous episodes of pancreatitis or abdominal trauma, as well as being haemodynamically stable on admission. Data regarding patient demographics, clinicopathological characteristics, and adverse events were collected.
Patients also had to have undergone EUS-guided cystogastrostomy over a 5-year period (2018 to 2022) at our interventional endoscopy unit. All cystogastrostomy and stent insertions were placed by a single surgeon trained in EUS. The indications varied amongst this case series with majority of patients (n = 6) with severely symptomatic pseudocysts and 1 gastric outlet obstruction (Figure 1). Stents were inserted in all cases, which were performed under general anaesthetic and antibiotics on induction.
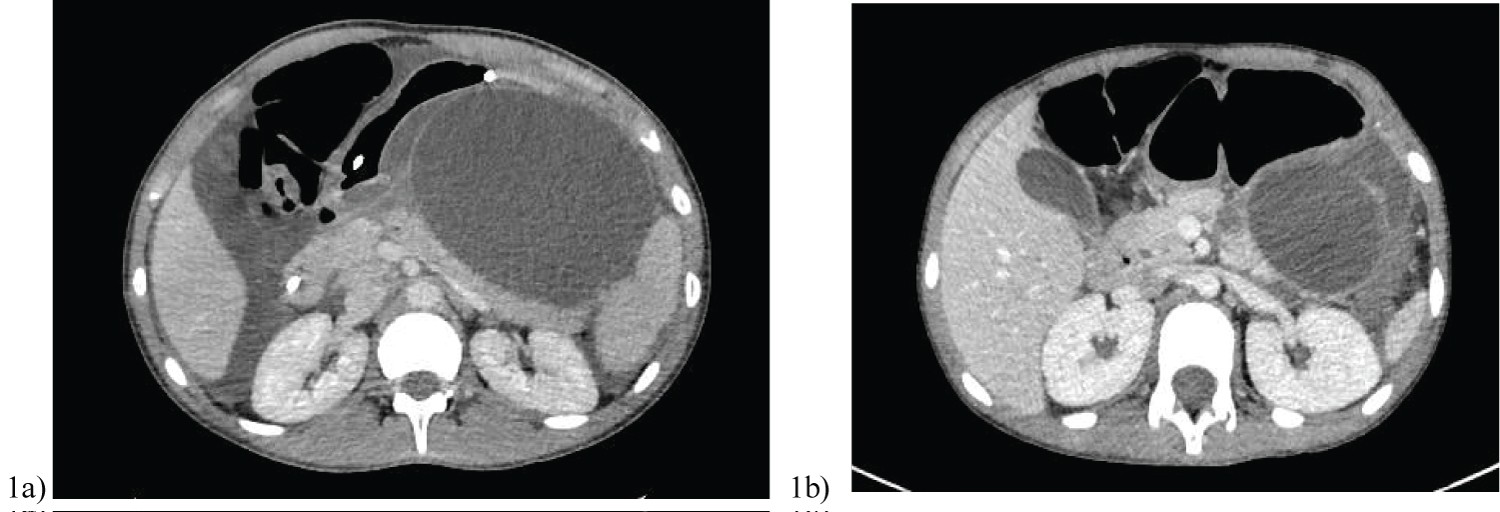 Figure 1: CT - angiography, CT - perfusion and cerebral angiography before endovascular treatment. A) CT - angiogram showing AVM and aneurism localization; B) CT - prefusion showing hyperperfusion zone in AVM and high-flow drainage vein; C) Cerebral angiography showing AVM of the right fronto-parietal region and the proximal flow - related aneurysm on the right terminal ACA afferent.
View Figure 1
Figure 1: CT - angiography, CT - perfusion and cerebral angiography before endovascular treatment. A) CT - angiogram showing AVM and aneurism localization; B) CT - prefusion showing hyperperfusion zone in AVM and high-flow drainage vein; C) Cerebral angiography showing AVM of the right fronto-parietal region and the proximal flow - related aneurysm on the right terminal ACA afferent.
View Figure 1
Endoscopic ultrasound (EUS) GF-UC140-AL5 (Olympus of America, Center Valley, PA, USA) scopes were used in all 7 cases. Firstly, the collections were analysed with EUS; the colour doppler technique identified overlapping vessels and then confirmed feasibility of the collection’s proximity to stomach. In case 6, the unilocular pseudocyst was identified between the neck and tail of pancreas, with the duodenal window to the collection more feasible in terms of physiological drainage.
In Figure 2a, a 19-gauge Cook needle (Cook, Bloomington, IN, USA) was then introduced into the lesser sac collection and a large volume of clear pancreatic fluid aspirated. A 0.035 soft Jag wire with subsequent cystotome to puncture across the gastric wall was performed. A second wire was placed into the cystotomy with a 7 French 4 cm double pigtail stent deployed (Figure 2b). Figure 3 demonstrates the cystoenterostomy performed in case 6, with stent position in duodenum. With EUS guidance, the entire procedure was performed under direct vision, thus preventing vascular injury. Post discharge, all patients were reviewed in hepatobiliary surgery outpatient clinic for assessment of symptom resolution.
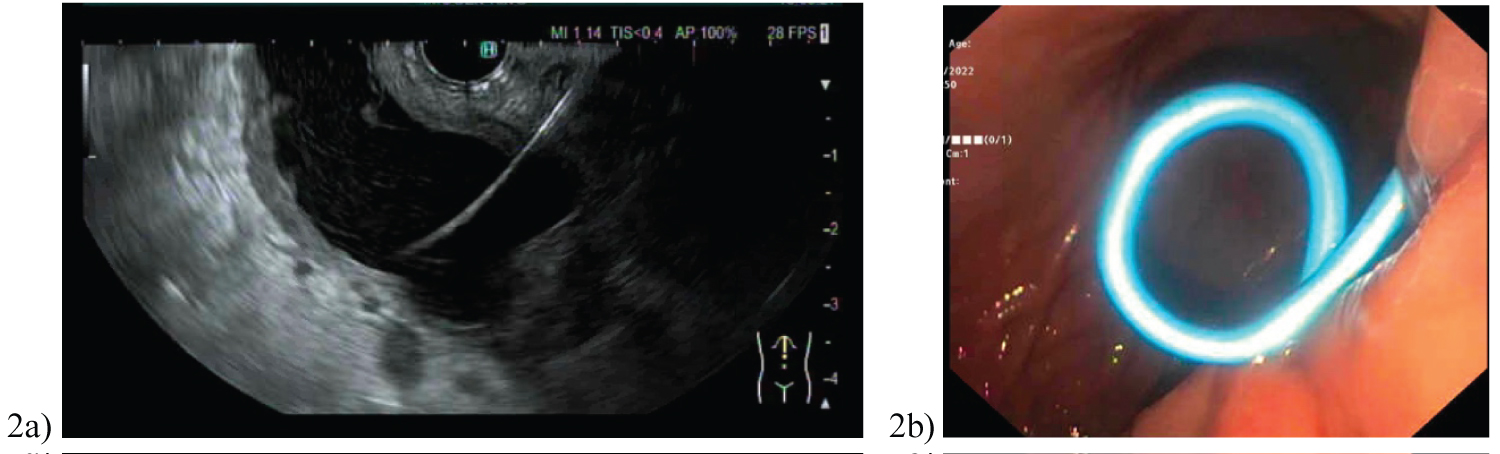 Figure 2: Superselective angiography: Determined by the right recurrent artery of Heubner and normal perforating arteries extending from the aneurism neck.
View Figure 2
Figure 2: Superselective angiography: Determined by the right recurrent artery of Heubner and normal perforating arteries extending from the aneurism neck.
View Figure 2
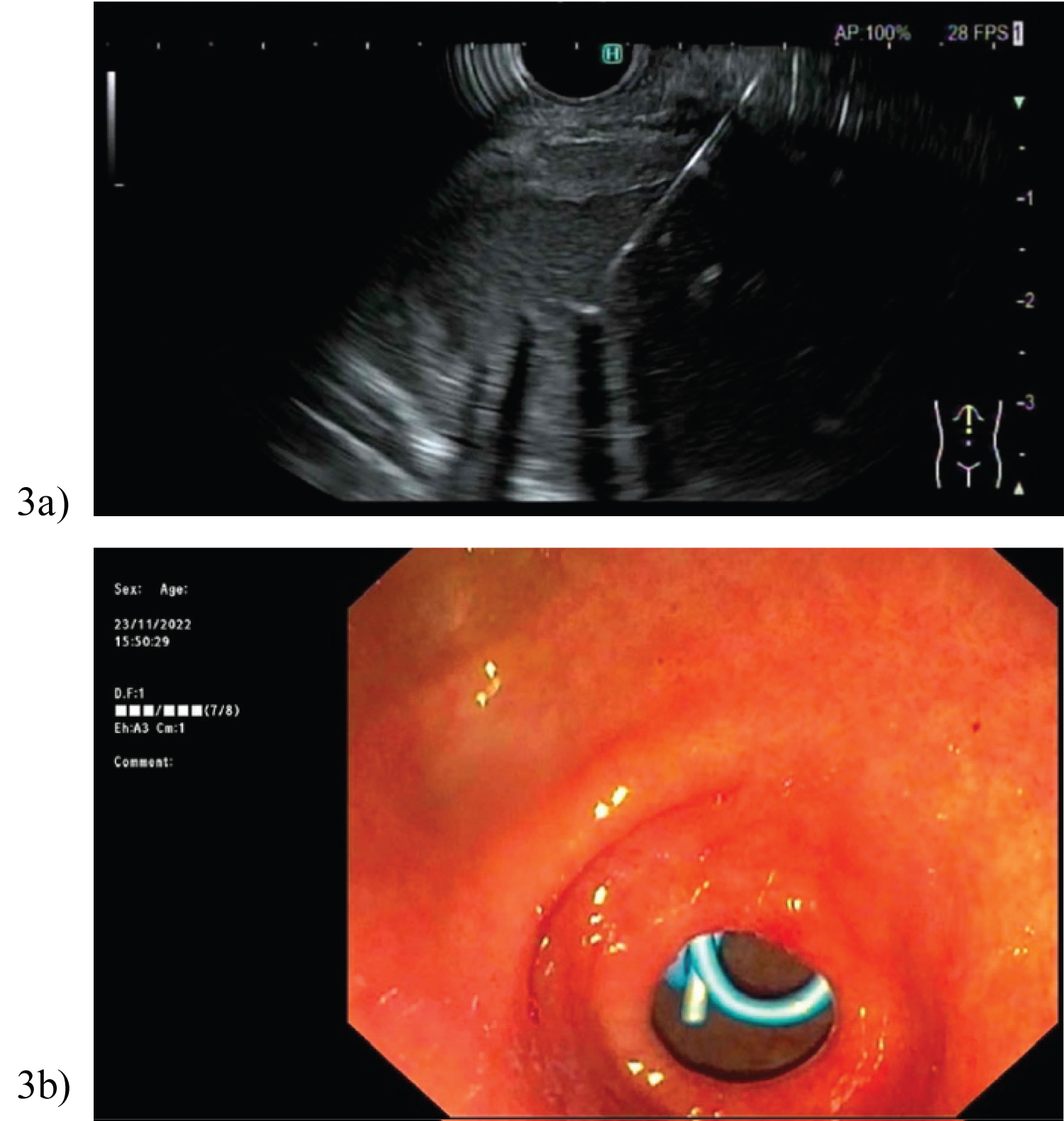 Figure 3: Supreselective angiography showing AVM node and embolized fistulous part of the AVM.
View Figure 3
Figure 3: Supreselective angiography showing AVM node and embolized fistulous part of the AVM.
View Figure 3
All 7 patients included in this series were well with no comorbidities or previous abdominal surgery; and haemodynamically stable at time of injury. Table 1 is a summary of their clinicopathological characteristics. The average age was 14 (age range 5-34 years), with majority of patients being female. Acute traumatic pancreatitis was confirmed with an elevated lipase ranging from 1700-12385 U/L, as well as a computed tomography (CT) showing an AAST Grade IV pancreatic neck transection (Figure 4). Case 4 and 5 also had additional organ injuries; ranging from grade I-II splenic and liver lacerations respectively.
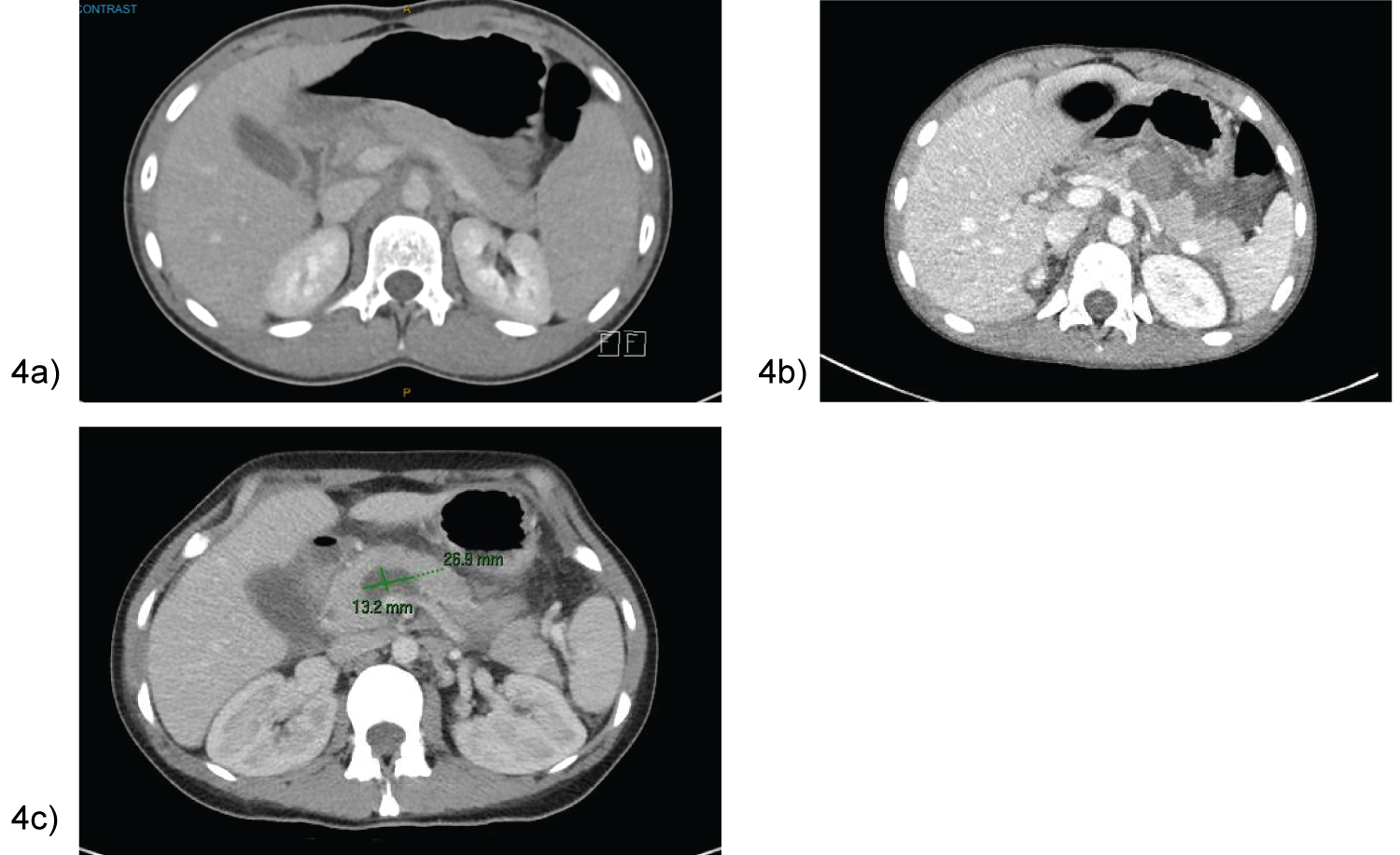 Figure 4: Control cerebral angiography showing embolized AVM, and flow - related aneurysm.
View Figure 4
Figure 4: Control cerebral angiography showing embolized AVM, and flow - related aneurysm.
View Figure 4
Table 1: Patient clinicopathological characteristics. View Table 1
Initial management included resuscitation, analgesia, and gastrointestinal rest with nil orally. 6 out of 7 patients had ERCP, with 5 being early ERCP for management of severe pain. Case 7, our only adult patient, did not have an ERCP as her symptoms were well controlled with patient controlled analgesia (PCA). Transpapillary drainage was successfully performed in 5 out of 6 ERCP patients, with cannulation and stent insertion of the transected pancreatic duct.
However on surveillance CT/magnetic resonance imaging (MRI) imaging, the MPD stent in all 6 patients were unable to prevent pancreatic leak, with further collections/pseudocyst in the lesser sac (Figure 1). The average size of the pseudocysts prior to EUS transmural drainage was 9.9 cm.
1 adverse event identified in our case series occurred with transpapillary drainage in case 2. She was re-admitted day 11 post ERCP MPD stent insertion with worsening nausea and epigastric pain. CT demonstrated a 14 × 8 cm peripancreatic collection causing gastric outlet obstruction (Figure 1b), with stent migration to descending colon. Close serial examinations were performed to ensure no stent perforation, with subsequent successful EUS cystogastrostomy.
2 patients required repeat EUS, with Case 6 undergoing a further 2 transmural drainages on day 19 and 34 post injury. This pseudocyst likely recurred as initial transmural drainage day 12 post injuries, was via cystoenterostomy. Post initial cystogastrostomy, the patient developed worsening abdominal pain and fevers. MRI pancreas demonstrated an irregular Y-shape like gas-containing pseudocyst (38 × 40 × 49 mm) with extension into left upper quadrant (33 × 41 × 70 mm) and gastrohepatic ligament (21 × 21 × 18 mm) (Figure 5). On second EUS cystogastrostomy, pus was identified emanating from the 2 appropriately sited stents on the lesser curvature of the stomach. Stent dilatation was performed up to 10 mm, and a third double pigtail stent inserted to improve drainage.
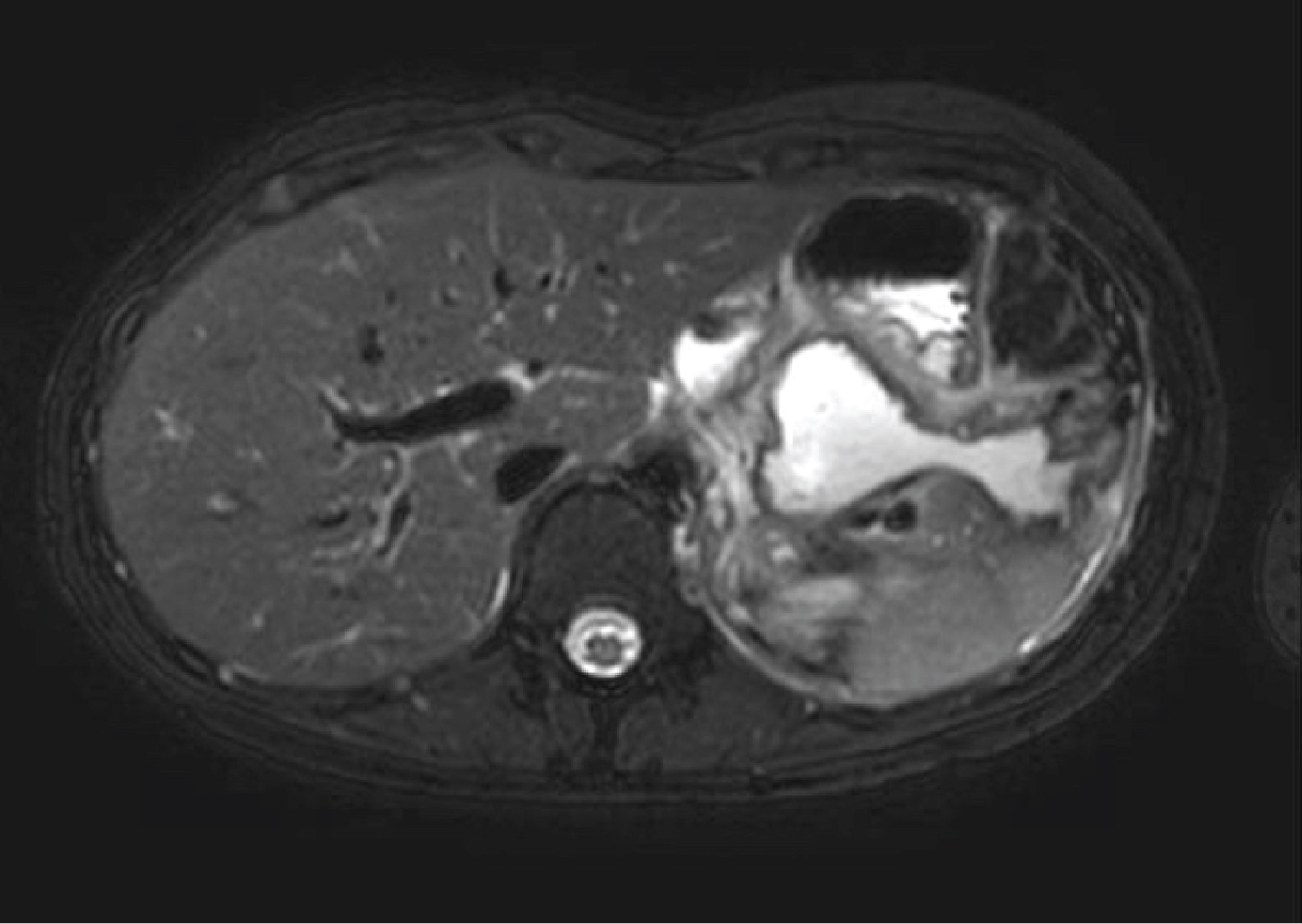 Figure 5: Control cerebral angiography showing embolized AVM and flow-related aneurysm. Left ICA. Blood flow in ACoA preserved.
View Figure 5
Figure 5: Control cerebral angiography showing embolized AVM and flow-related aneurysm. Left ICA. Blood flow in ACoA preserved.
View Figure 5
Case 7 had a non-interventional EUS day 16 post injury as the peripancreatic collection was a heterogenous nature and not in an ideal drainable position. Planned elective cystogastrostomy occurred 37 days post injury with good effect. Both Case 6 and 7 made a rapid recovery.
Majority of patients were asymptomatic with no signs of pancreatic endocrine or exocrine insufficiency over the course of a 10-36 month follow-up. All patients had significant improvement in pseudocyst size as demonstrated in Figure 6a. Case 1’s MRI at 36 months depicted a normal head and uncinate process, with distal atrophy of the pancreas and MPD (Figure 6b).
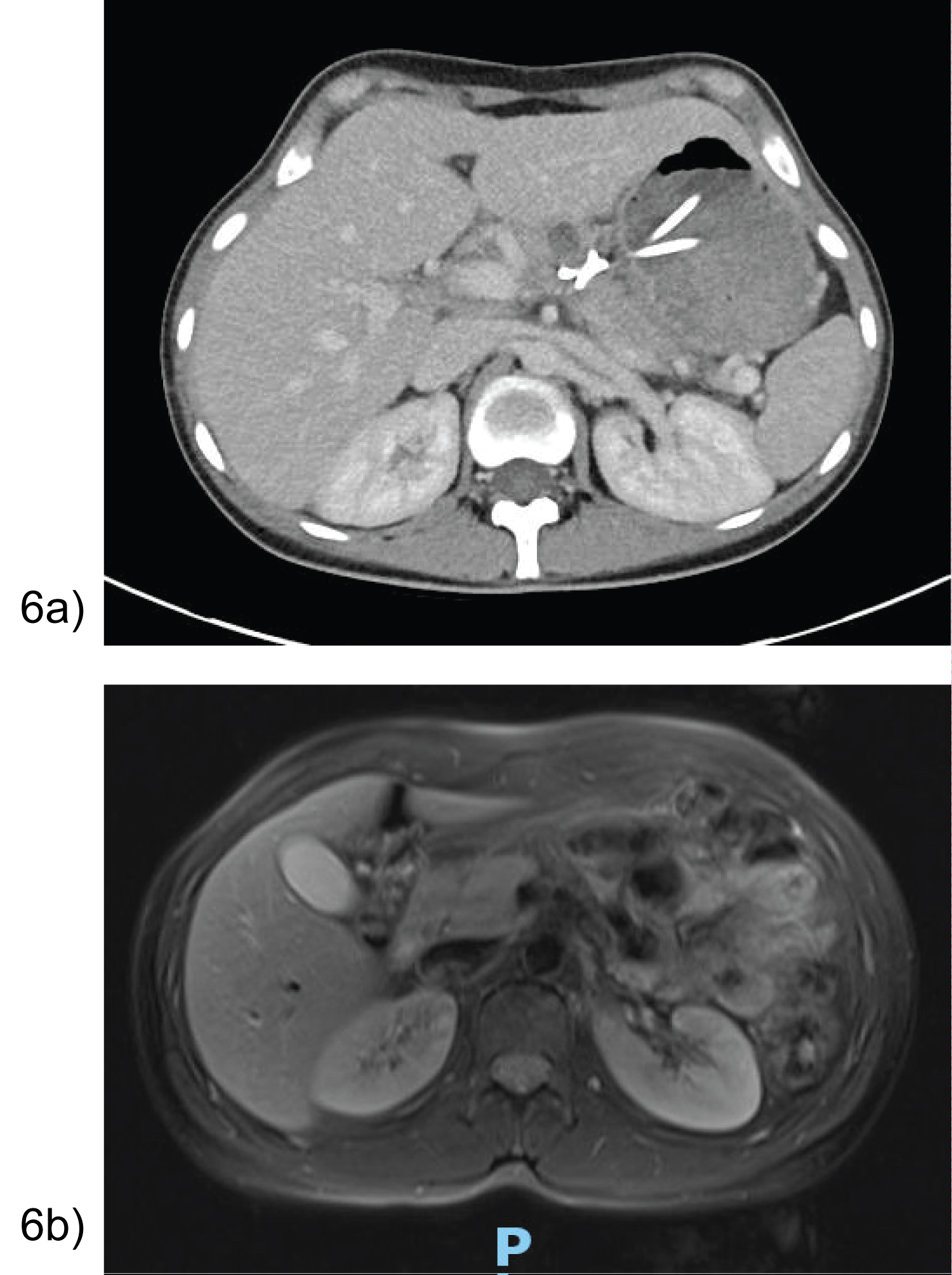 Figure 6: (a) Case 7- CT day 15 post cystogastrostomy showing stents in position with interval decrease in peripancreatic collection; (b) Case 1- MRI 36 months post EUS cystogastrostomy with distal pancreatic atrophy and duct.
View Figure 6
Figure 6: (a) Case 7- CT day 15 post cystogastrostomy showing stents in position with interval decrease in peripancreatic collection; (b) Case 1- MRI 36 months post EUS cystogastrostomy with distal pancreatic atrophy and duct.
View Figure 6
However, Case 2 failed to clinically improve; with 2 years of chronic pain episodes affecting quality of life and requiring multiple admissions. In April 2022, a laparoscopic Warshaw procedure-distal pancreatectomy with splenic preservation was performed. Intra-operative findings identified inflammatory adhesions and complete pancreatic transection left of superior mesenteric vein and portal vein junction. She had an uneventful recovery with discharge 5 days later. On review, her symptoms had resolved and she was back at school.
Pancreatic trauma has a 4-5% occurrence with MPD disruption an even rarer 0.3-0.7% incidence [1]. Its management is an ongoing challenge in both paediatric and adult patients with variations in clinical practice [1,3,5,7,8,11]. As a retroperitoneal organ, neck injury in particular, occurs due to forceful compression against the posterior vertebral spine. This mechanism is depicted in Figure 5, with majority due to handlebar injuries (n = 4). Children and thin adults display the characteristics of a flatter diaphragm, thinner abdominal wall and high costal margins- putting them at higher risk for pancreatic trauma [2]. This was exemplified in our case series, including our low body mass index (BMI) adult patient.
In 1990, the AAST classified pancreatic injuries into five grades from I-V, based on the extent of parenchymal injury and haematoma [2]. Grade I and II injuries were mild with no MPD disruption, whilst grade III-V traumas involved a degree of MPD disruption with higher-risk complications such as haemorrhage from peripancreatic vessels, pancreatic fistulas, pseudocysts, and intra-abdominal abscesses [2]. This was also associated with significant morbidity and mortality [2,8]. All patients in this case series sustained traumatic grade IV injuries with proximal neck transection and MPD disruption; with subsequent pseudocyst/peripancreatic collection formation.
Albeit haemodynamically stable, our patients presented with classic signs of traumatic pancreatic injury- severe epigastric tenderness, elevated lipase, and CT confirmation of pancreas pathology [2,11,12]. However, in case 3, the CT was inconclusive regarding MPD disruption; likely due to her low BMI and lack of retroperitoneal fat. Magnetic retrograde cholangiopancreaticography (MRCP), reported in the literature as the next appropriate radiological investigation [1,12], was also unable to delineate the integrity of Case 3’s MPD despite evidence of pancreatic hypoattenuation and extensive free fluid. With rising inflammatory markers and a florid SIRS response, further ERCP could exacerbate her pancreatitis. Conversely, the option of a damage control laparotomy would require external drainage with high risk for pancreatic fistulas and ongoing leak [1,7]. Hence the decision for a short trial of observation and total parental nutrition was made.
Historically, grade I-II injuries were conservatively managed as undisputed standard of care [2-4]. However, debate ensued with grade III-V traumas as early opinion dictated immediate surgical drainage or resection [1,2,11]. Even in the paediatric population, many recommended distal pancreatectomy or pancreatico-jejunostomy depending on MPD disruption location [12,13]. Iqbal, et al.’s analysis of 57 paediatric patients post resection and 95 non-operative patients found a shorter diet upgrade timeframe and lower rate of pseudocyst formation in the former group [13]. The 2009 Eastern Association for the Surgery of Trauma guidelines also recommended early intervention compared to observation for peripancreatic collections due to the risk of infection; demonstrating a shorter length of stay (LOS) and fewer complications [12].
This management pathway remained controversial with wide practice variability found in surveys of surgeons. Many found it aggressive with long term complications of pancreatic endocrine and exocrine insufficiency not fully appreciated [1,11]. Subsequently, a study by Burnweit, et al. advocated percutaneous drainage as an alternative, with early promising outcomes [7]. In the retrospective review by Wales, et al., 9 paediatric patients with MPD disruption were treated non-operatively [14]. Similar to our patients, 44% developed pseudocysts; with 3 out of 4 undergoing percutaneous external drainage [14]. The median length of stay (LOS) was 24 days, with no long-term complications of pancreatic insufficiency [14]. This is in stark contrast to our patients who all underwent internal drainage; with a median LOS of 9 days and equivalent long-term outcomes.
Rosenfeld, et al. was the first to utilise ERCP as a diagnostic tool to evaluate duct injury, as well as minimally invasive therapeutic management [9]. The ERCP stent would block the disrupted duct and convert the high-pressure pancreatic duct system into a low pressure one, draining pancreatic enzymes into the duodenal papilla [9]. It was also useful in late complications such as strictures and fistulas [8]. This is exemplified in our paediatric cases who all underwent ERCP; 5/6 of them being successful. We surmise failure of stent insertion occurred in case 1, due to a significantly shortened proximal pancreatic duct. Her ERCP procedure was also 14 days post injury, as compared to the other successful ERCPS which were performed within 1-3 days post injury. Hence having failed trans-papillary drainage, transmural drainage via EUS cystogastrostomy was performed. The outlier to our management pathway was adult patient case 7; conservatively managed without ERCP as discussed above.
The literature describes other ERCP-related complications such as pancreatitis exacerbation, strictures, and converting partial MPD disruption into a complete one [8,9]. Case 2 was the only complication (Clavien-Dindo grade 3b) encountered in our case series. She initially underwent ERCP day 3 post injuries, with discharge 2 days later. However, she represented with ongoing peripancreatic collections and colonic stent migration. Fortunately, no iatrogenic perforations occurred, and the stent subsequently passed with no further intervention required.
EUS-guided transmural drainage for pseudocyst was first described in 1992 [15], and it is now the gold standard treatment based on safety and efficacy in the proven setting of atraumatic pancreatitis [16,17]. In the past, open pseudocyst cystogastrostomy with or without pancreas resection in a physiologically unoptimized patient had risks. Endoscopic management was demonstrated to have successful outcomes similar to surgical management, as well as the advantage of shorter length of stay and reduced cost [16,18]. In our case series of trauma mechanism-related peripancreatic collections/pseudocysts, we found EUS transmural drainage avoided operative management in 6 out of the 7 patients. Case 2 who underwent a delayed laparoscopic distal pancreatectomy was physiologically optimised, which we believe contributed to the minimally invasively operation’s success. This management option was also effective in the multi trauma setting, evident in Case 4 and 5. We also note no complications of stent migration when comparing EUS transmural drainage to ERCP transpapillary drainage. In terms of cystoenterostomy vs cystogastrostomy drainage, only 1 was performed for the former option (Case 6) which subsequently required further cystogastrostomy drainage. This may imply superiority with trans-gastric stent placement compared to trans-duodenal stent placement.
The strength of this case series was the encompassment of both paediatric and adult patients, speaking to its generalizability for these demographic groups in pancreatic trauma. The limitations of this case series- single centre institution aspect, retrospective study design, and small sample size result in statistically insignificant conclusions. However, majority successfully underwent transmural drainage via EUS-guided cystogastrostomy, with a clinically positive outcome and no adverse events.
Although management of pancreatic pseudocysts with transmural drainage is a gold-standard intervention, this is not yet a proven strategy in the indication of traumatic MPD disruption. Due to the rarity of blunt pancreatic traumas, the literature has been limited. However, this case series demonstrates the potential for EUS-guided transmural drainage in the management pathway of traumatic pancreatic pseudocysts in stable patients, with low morbidity. Further studies with long-term surveillance are required to support the use of cystogastrostomy in severe pancreatic trauma.
All authors have no conflict of interest in the production of this manuscript. No financial or material support for the research and the work reported was obtained. Assistance of medical writing experts was not sought beyond the authors.
The authors listed have no sources of support or funding received for this work from any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; and other(s).
Authors 1, 2, 3 were involved in data collection. Author 1 wrote the manuscript with subsequent editing from Authors 2, 4, 5.