Group A rotavirus is the leading cause of diarrhea hospitalization among children worldwide. Most fatal rotavirus-associated diarrhea cases among children under 5 years occur in Africa and Asia. We investigated the molecular epidemiology of circulating rotavirus strains among children less than 5 years in western Kenya to provide baseline data on the prevalence of each genotype prior to the introduction of monovalent rotavirus vaccine in Kenya.
From January 2010 to December 2013, stool samples were collected from 1677 children with acute gastroenteritis. All specimens were transported to Kenya Medical Research Institute, Center for Global Health Research, Enterics Laboratory and tested for rotavirus antigens using enzyme immunoassay. Rotavirus dsRNA was extracted from 234 simple randomly selected EIA positive stool samples using QIAamp viral RNA mini kit and tested by semi-nested RT-PCR for G and P genotypes using type-specific primers. The PCR products were analyzed by gel electrophoresis. Chi-square test was used to test the association between rotavirus genotypes and age.
Of the 1677 stool samples tested, 401 (23.9%) were positive for group A rotavirus antigen. Of the 234 rotavirus dsRNA extractions analyzed by PCR, 219 (93.6%) and 193 (82.5%) typed positive for at least one of the VP7 genotypes (G type) and VP4 genotypes (P types), respectively. Of the typeable, 19 were mixed G types and P types. However, 15 VP7 and 41 VP4 were nontypeable. The predominant genotypes detected included G1 (30%), G9 (27%), G8 (10%) and G3 (9%) for the G types, and P[8] (33%) and P[6] (30%) for the P types. The predominant combinations were: G1P[8] (15%), G9P[8] (12%) and G3P[6] (8%) which combined accounted for 35% of the genotypes detected.
This study demonstrated the genotype diversity and dominance of G1, G3, G8 and G9 in combination with P[6] and P[8] as the most common genotypes associated with rotavirus gastroenteritis in this population. Continuous surveillance is necessary to monitor the effectiveness of the vaccine and shifts among the circulating genotypes in this region.
Acute gastroenteritis, Rotavirus, Enzyme immunoassay, Polymerase chain reaction, Genotype, Rotavirus vaccine
GEMS: Global Enterics Multisite Study; GAVI: Global Alliance for Vaccine and Immunization; HDSS: Health and Demographics Surveillance System; KEMRI: Kenya Medical Research Institute; CDC: Centers for Disease Control and Prevention; AGE: Acute Gastroenteritis; EIA: Enzyme Immunoassay; RT-PCR: Reverse Transcription-Polymerase Chain Reaction; dsRNA: double stranded Ribonucleic acid; cDNA: complementary Deoxyribonucleic Acid; WHO: World Health organization; MoH: Ministry of Health, Kenya
Diarrhea is one of the major causes of infant morbidity and mortality worldwide. Of deaths caused by infectious diseases, diarrheal disease is the second most common and contributes to approximately 9% of deaths among children below 5 years of age, most of which occur in Sub-Saharan Africa and southern Asia [1]. Before the implementation of the rotavirus vaccines, rotavirus was the leading cause of diarrhea hospitalization among children worldwide [2]. Most moderate to severe diarrhea cases among children under 5 years attributed to rotavirus infection occur in Africa and Asia [3]. Despite the decline in rotavirus related deaths from 528,000 in 2000 to 215,000 in 2013, the mortality rates remain high in sub-Saharan Africa and Asia [2,4]. A previous study in Kenya found that rotavirus infections caused 19% of hospitalizations and 16% of clinic visits for diarrhea among children < 5 years of age and cost the health care system $10.8 million annually, while the overall annual burden of rotavirus-associated mortality in Kenya was estimated to be 68 deaths per 100,000 children < 5 years [5]. Recent evidence from the Global Enterics Multisite Study (GEMS), combined with the burden of diarrheal disease analysis in western Kenya, reported over 500 hospitalizations for children under 5 years per 100,000 person years and a mortality of 136 deaths per 100,000 person years all attributable to rotavirus gastroenteritis. These studies reported that rotavirus is the most common cause of moderate-to-severe diarrhea during the first year of life [3,6]. However, with increased uptake of rotavirus vaccine, there is optimism that the decision by the Global Alliance for Vaccine and Immunization (GAVI) to support making rotavirus vaccines available to the world's poorest countries will reduce the burden of the disease substantially as has been observed in countries which introduced the vaccines earlier [7].
Rotaviruses are divided into 7 groups (A to G) based on group specificity which is predominantly conferred by VP6 whereas the outer-capsid proteins VP4 and VP7 specify rotavirus serotype specificities [8]. Most human infections are caused by group A rotaviruses, which can further be differentiated into 27 G types and 35 P types by VP7 and VP4 genotyping, respectively [9]. The host antibody response is normally directed toward the viral surface antigens. A global survey showed that G1, G2, G3 and G4 were the most common worldwide genotypes of rotaviruses while G8 was relatively high in Africa [10]. Although antibodies to rotavirus antigens cross-react, knowledge of the distribution of G and P genotypes, including detection of emerging genotypes, is potentially crucial to rotavirus vaccination programs [11].
There are two rotavirus vaccines currently available for use globally, a pentavalent bovine human reassortant vaccine (RotaTeq®, Merck & Co., Inc. West Point, PA) and a monovalent vaccine based on an attenuated human rotavirus strain (Rotarix™, Glaxo-SmithKline Biologicals, Rixensart, Belgium). Both vaccines were shown to be safe and effective in clinical trials although more modest efficacy was observed in Africa and Asia compared with the Americas and Europe [12,13]. In July 2014, Kenya introduced Rotarix® into its national immunization program. The vaccine was approved for use in infants 6 weeks to 24 weeks of age. It is well-tolerated in infants and elicits improved immunologic responses which include rotavirus-specific IgA antibodies [14]. Like RotaTeq® which is designed to protect against rotavirus infection in young children caused by genotypes G1, G2, G3, G4 and P[8], Rotarix® provides homotypic protective immunity to G1P[8] strains and heterotypic immunity against non-G1P[8] genotypes [14,15].
Comparison of rotavirus strains pre and post introduction of the vaccine into routine infant immunization programs will help in future policy-making to guide the use and distribution of the available rotavirus vaccine types. This is particularly important in settings like Sub-Saharan Africa where pre-vaccine rotavirus strains are quite diverse [16,17]. In the current study, we investigated the molecular epidemiology of circulating rotavirus strains among children in western Kenya to provide the baseline data of the genotypes distribution prior to the introduction of rotavirus vaccine.
Rotavirus surveillance was conducted from January 2010 to December 2013 in Siaya County, Nyanza region, western Kenya within the Karemo and Gem areas of the Health and Demographics Surveillance System (HDSS) program, which was implemented by the Kenya Medical Research Institute (KEMRI) in collaboration with the Centers for Disease Control and Prevention (CDC). This area is holoendemic for malaria [18,19], has a high prevalence of HIV/AIDS [20], high levels of malnutrition [21], and has limited access to piped water systems. In Kenya, this area has among the highest rates of infant and child mortality rates of 87 deaths/1000 live births and for children < 5 years, mortality ratio was 171 deaths/1000 live births in 2012 [19].
Stool samples were collected from inpatient and outpatient children aged < 5 years who presented at one inpatient and two outpatient study health facilities with acute gastroenteritis (AGE) from January 2010 to December 2013. AGE was defined as ≥ 3 loose stools and/or ≥ 1 episode of unexplained vomiting followed by loose stool within a 24-hour period beginning no more than 7 days before the visit. Stool sample collection was performed as previously described [6]. Briefly, stool was collected in a plastic diaper from which at least 2 ml of stool was scooped into a specimen container using a sterile spatula. The stool was collected within 48 hours of admission to the hospital or visit to the health facility. In the outpatient facilities, a community interviewer would follow-up with the mothers at home to collect the stool produced before 48 hours, if the child met the criteria but was unable to provide a stool sample during the outpatient visit.
All stool samples were transported on the same day of collection to KEMRI, Center for Global Health Research, Enterics Laboratory, on ice packs. Rotavirus group A antigen was detected in stool specimens using a solid-phase sandwich-type enzyme immunoassay (EIA) kit (Premier® Rotaclone®, Meridian Bioscience, Cincinnati, Ohio, USA) according to manufacturer's instructions. EIA positive stool specimens were shipped to the Rotavirus Regional Reference Laboratory, South African Medical Research Council/Diarrheal Pathogens Research Unit, Department of Virology, Sefako Makgatho Health Sciences University in Pretoria, South Africa for molecular characterization.
Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected for Rotavirus dsRNA extraction using the QIAamp viral RNA mini kit according to the manufacturer instructions. The extracted dsRNA was analyzed by semi-nested RT-PCR for genes specifying G and P genotypes using type-specific primers as previously described [22,23]. Consensus primers sBeg9 and End9 were used in the first round of PCR to amplify the full-length VP7 gene (1062 bp). The cDNA was used for G typing using primers End9, aAT8v, aBT1, aCT2, aDT4, mG3, mG9, mG10 and G12 for G1, G2, G3, G4,G8, G9, G10 and G12 genotypes, respectively [23,24]. Primers, Con2 and Con3, were used in the RT-PCR to amplify the partial-length of VP4 gene of 876 bp. Genotyping was done using a mixture of primers consisting of Con3 and primers 1T-1, 2T-1, 3T-1, 4T-1, 5T-1, mP[11] and P[14] for P[8], P[4], P[6], P[9], P[10], P[11] and P[14], respectively [22,25]. All PCR products were analyzed by electrophoresis in 2% agarose gels with 0.5 µg/ml ethidium bromide at 90 volts in 1X TBE buffer and visualized with a gel documentation system under UV illumination.
Data were stored and managed using a Microsoft SQL Server 2008 database. Statistical analysis was done using Stata 12 (Stata Corporation, College Station, TX). Chi-square statistics was used to test the association between rotavirus genotypes and age. For all analyses, a p-value of less than 0.05 was considered statistically significant.
We collected 1677 stool specimens from eligible children from January 2010 to December 2013 and tested them for rotavirus group A antigen. Children below 11 months of age were the majority (n = 1044, 62.2%), and 645 (56.4%) were male. Overall, 401 (23.9%) were positive for rotavirus by EIA (Table 1). The difference in rotavirus detection by gender groups was not significant (p = 0.641). However, there was a statistically significant difference in rotavirus positivity by age groups (p < 0.0001) with the higher being 28.2% (124 of 440) among children below 5 months and the lower being 11.2% (57 of 270) among children between 24-59 months old. Similarly, the proportion of rotavirus positive cases was higher in 2010 and 2011 (23.9% and 26.1% respectively) and lower in 2012 and 2013 (22.4% and 21.7% respectively). However, the difference in rotavirus positivity by year was not statistically significant (p > 0.51).
Table 1: Comparison of rotavirus EIA tests results by age, gender and year among children less than 5 years of age presenting with AGE in Siaya, western Kenya, 2010 to 2013. View Table 1
Of the 234 stools randomly selected for genotyping which consisted of 166 (70.9%) from inpatient and 68 (29.1%) from outpatient, 200 (85.5%) were positive for one VP7 genotype (G type), 19 (8.1) were mixed G types and 15 (6.4%) were nontypeable (GNT) (Table 2). VP4 (P-types) genotyping detected 174 (74.4%) single P types, 19 (8.1%) mixed P types and 41 (17.5%) nontypeable (P[NT]). The sequence analysis data for the nontypeable (NT) strains was not reported in this paper. Overall, the predominant genotypes detected included G1 (29.9%) and G9 (26.9%) for the G types, and P[8] (32.9%) and P[6] (30.3%) for the P types. Mixed infection with G1 and G8 was observed among the G types but was more common among the P types with P[6]/P[8] dominating. Up to three G and P mixed genotypes were detected and, consisted of 9 and 4 different combinations respectively.
Table 2: Rotavirus G or P types among children under 5 years of age with AGE in Siaya, Western Kenya (2010-2013). View Table 2
The distribution of the G types did not vary substantially with age, and G1, G9, P[6] and P[8] strains remained predominant across all the age groups (Table 3). However, there was notable variation in the distribution of the P types by age category with P[6] and P[8] being more dominant in the first 3 age groups than the older group while P[4] and P[NT] dominated the older group. Most rotaviruses (G and P types, 93.7%) were detected in children below 2 years of age with 75.8% G types and 75.9% P types in children less than 1 year of age.
Table 3: Rotavirus G or P-types by age among children under 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013). View Table 3
The distribution of rotavirus G and P types varied considerably over the 4 years of the study. For G types, G1 and G9 remained the dominant strains throughout the study period both contributing to a total of 133 (56.8%) of the samples tested (Figure 1A). There was a steady increase of G1 from 17.9% in 2010 to 46.4% in 2013. G3 and G8 were among the most prevalent strains in 2010 but disappeared in 2011 (0%) with an increase of G9 and G12 genotypes. However, G3 and G8 re-emerged in 2012 with a decrease in G12 strains over the same period. A decrease of G9 was also observed in 2013 (Figure 1B). Mixed G types were more common in 2010 (20.9%) than in the subsequent years of the study period (< 5%).
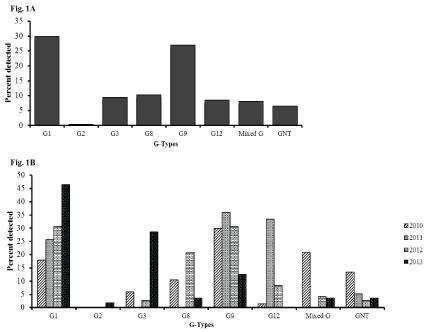 Figure 1: Rotavirus G types among children less than 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for genes specifying G and P genotypes. Figure 1A shows the overall percentage of the G-types. Figure 1B shows their distribution by year.
View Figure 1
Figure 1: Rotavirus G types among children less than 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for genes specifying G and P genotypes. Figure 1A shows the overall percentage of the G-types. Figure 1B shows their distribution by year.
View Figure 1
Among the P types, P[6] and P[8] were the dominant strains both contributing to over 50% of the rotavirus genotypes each year and 63% overall during the study period. Mixed G types accounted for 8% of the genotypes while approximately 17% were nontypeable (Figure 2A). P[4] strain was observed in > 11% of rotavirus positive specimens in 2010 and 2012, but accounted for only 5% and 2% of strains in 2011 and 2013 respectively. An emergence of P[14] was observed in 2012 which increased to 3% in 2013 (Figure 2B).
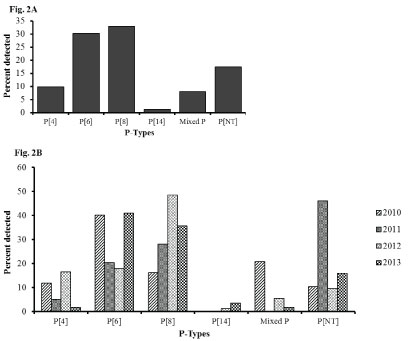 Figure 2: Rotavirus P types among hospitalized children under 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for genes specifying G and P genotypes. Figure 2A shows the overall percentage of the P-types. Figure 2B shows their distribution by year.
View Figure 2
Figure 2: Rotavirus P types among hospitalized children under 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for genes specifying G and P genotypes. Figure 2A shows the overall percentage of the P-types. Figure 2B shows their distribution by year.
View Figure 2
In G-P combination analysis, 15 binary characteristics and 31 (13%) mixed genotypes were obtained. However, 56 (24%) were nontypeable for either G or P types. The top three predominant combinations detected during the study period were: G1P[8] (15%), G9P[8] (12%), and G3P[6] (8%) contributing to a combined 35% of the genotypes detected. Mixed genotypes contributed to (13%) of the infections (Table 4). There was a steady increase in G1P[8], G9P[8] with a decrease in G9P[6] over the years of the study (Figure 3). Notably, G1P[6] and G3P[6] became equally predominant strains in 2013. Mixed infection was common in 2010 (33%) and steadily decreased to 5% in 2013.
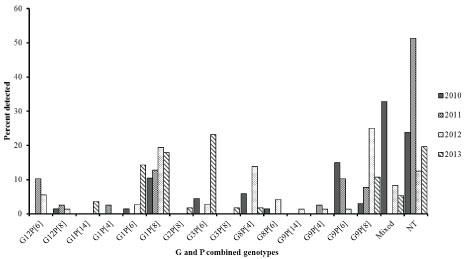 Figure 3: Distribution of Rotavirus genotypes by year among children under 5 years of age with acute gastroenteritis in Siaya, western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for G-P binary genotype characteristics.
View Figure 3
Figure 3: Distribution of Rotavirus genotypes by year among children under 5 years of age with acute gastroenteritis in Siaya, western Kenya (2010-2013): Stool samples were stratified by year and using simple random selection, 234 of 401 rotavirus EIA positive specimens were selected and analyzed for G-P binary genotype characteristics.
View Figure 3
Table 4: Prevalence rotavirus genotypes types among children under 5 years of age with AGE in Siaya, Western Kenya (2010-2013). View Table 4
When analyzed by age groups, G1P[8] and G9P[8] were found to be common among children of age 6-11 and 12-23 months old, less frequent in 0-5 months of age and none in the > 23 months age group. The proportions of G1P[8] and G9P[8] was higher among children of 6-11 months (18.7% and 15.4% respectively) compared to other age groups. Other predominant circulating strains included: G3P[6], G8P[4] and G9P[6] all contributing to 20% of genotypes detected across all the age groups (Table 5). Mixed infection was common across all the age groups but higher in upper age category (21% for 24-59 months old children). The overall prevalence of rotavirus infection among the upper age group was generally low.
Table 5: Rotavirus P-G Types combination by age among hospitalized children under 5 years of age with acute gastroenteritis in Siaya, Western Kenya (2010-2013). View Table 5
The median age of children found with G1P[8] and G9P[8], the predominant genotypes, was 8.6 and 8.5 months, respectively (Table 6). The severity of diarrhea did not vary substantially by infecting genotype. However, the median duration of diarrhea before seeking care, for the most prevalent genotypes, was 3 days whereby patients infected with G8P[4] and mixed P genotypes were more likely to present with more than 5 loose stools within 24 hours while the rest presented with 4 or less loose stools within the same duration. The number of diarrhea stools and vomiting episodes reported did not vary much among the genotypes detected. Similarly, the proportions of two genotypes (G1P[8], 72.2%; and G9P[8], 86.2%) were higher among the hospitalized children compared to the outpatients (data not presented). The same was observed for mixed genotypes.
Table 6: Frequency of rotavirus genotypes and severity of infection among hospitalized children under 5 years of age presenting with AGE in Siaya, Western Kenya (2010-2013). View Table 6
Two G types (G1 and G9) and 2 P types (P[8] and P[6]) were found to be the main genotypes circulating in this study area. Together, the above G types constituted 57% of all the cases tested for G genotype while the 2 P types constituted 63% of cases analyzed. G1P[8] (15%), G9P[8] (12%) and G3P[6] (8%) were the main circulating genotypes combination together contributing to 35% of AGE in Siaya county, consistent with previous studies done in Africa and Asia [26-29].
Previous studies have shown that following primary rotavirus infection, children develop immunity against subsequent rotavirus infection and rotavirus diarrhea [30,31]. We found that G1P[8] and G9P[8] were more dominant in age group 0-23 months, but not in the older age group (24-59 months) consistent with this observation even though fewer rotaviruses were detected and genotyped from the older age group.
Serotype diversity and reassortment between human and animal rotavirus strains has resulted in numerous G and P- types [32,33]. However, the common G (G1, G2, G3, G4 and G9) and P (P[8], P[6] and P[4]) types predominate in most studies all over the world [17,34,35]. In the current study, the genotypes detected were within this group whereby G1 and G9 were the most frequently detected genotypes contributing to 57% of rotavirus positive cases which is consistent with studies conducted in Africa [17,27]. The genotypes G1 was found more frequently in combination with P[8] and less with either P[6] or P[4] which is a common observation in many studies in Africa and globally [10,17]. Previously, G3 was infrequently detected in many countries in Africa except in the southern regions of Africa [36]. However, in recent years, it has become one of the dominant strains in many countries in the continent [17]. In our study, G3 was detected in 9% of rotavirus positive cases and mostly in combination with P[6].
Infection with G9 genotypes is becoming more frequently reported in many countries in Europe and Africa [10,27]. Unlike a recent study in Tanzania and some parts of Asia [29,37] where only 1 G9 genotype was found in combination with P[8], we found 29 (12%) cases with similar combination and 15 (6%) cases in combination with P[6]. G2 strain was found to be less common in East Africa compared G1, G8 and G9 strains [17]. Further, the study found that G2 strain was the third most common strain in the countries in the central and southern part of Africa and that its prevalence varied by seasonality, year and country [17]. Here, we detected G12 (9%) and G2 (< 1%) but no G4 genotypes. These findings indicate that the genotype combinations may vary geographically and chronologically; hence, continuous surveillance studies would be helpful to monitor shifting in the prevalence patterns and effectiveness of available vaccines.
Mixed infections were also detected in 17 of 221 (7.7%) G types and 16 of 221 (7.2%) P types. G1 was detected in all the mixed G types except in two samples with G8/G3 and G8/G9 infection. Among the P types, P6/P8 was the most common mixed type. Other mixed types included P4/P6 and P4/P8 detected in 1 sample each and P4/P6/P8 in 3 samples. Such frequency of mixed infection has been reported from previous studies [38-40] and may play a role in strain diversity due to recombination. Previous studies have reported failure of genotype specific PCR primers to amplify the VP7 and VP4 genes of the most common global strains including G1 and P8 [25,41]. In the current study, 55 (24.9%) were identified as nontypeable by RT-PCR. Nucleotide sequencing revealed a number of primer mismatch at the binding sites of both RT-PCR and genotyping primers (data not shown). The genetic variation at the primer binding sites was due to nucleotide substitution that may have affected the sensitivity of the assays. Nucleotide sequencing or use of more than one set of genotype-specific primers could resolve some of the nontypeable strains.
Not all the samples were sequenced due to limited resources. Interestingly, nucleotide sequence of nontypeable strains showed sequence identity of 89-99% with the African reference strains from the Genbank. Genomic analysis to monitor molecular evolution of these strains was not accomplished.
This study demonstrated the genotype diversity and dominance of G1 and G9 in combination with P[6] and P[8] as the genotypes associated with rotavirus gastroenteritis in this study population. Rotavirus vaccine Rotarix™ was recently introduced in Kenya in July 2014 and was included in the National Immunization Program. Rotarix™ vaccine elicits immune protection against rotavirus gastroenteritis due to G1P[8] strains and heterotypic immune protection other G and P types suggesting high coverage of the vaccine. However, whether the heterotypic immunity due to rotavirus vaccine will play a significant role in protection against rotavirus gastroenteritis caused by the diverse genotypes, including the dominant G9, circulating in this region and impede shift to uncommon genotypes is not clear. Thus, continuous surveillance is necessary to monitor the effectiveness of the vaccine and shifts among the circulating genotypes in this region.
The authors acknowledge the contribution of KEMRI staff in Enterics Laboratory, Data section and the hospital community where this work was performed. We acknowledge HDSS, the residents of Siaya County for their participation in this study, KEMRI Director, the Kenya Ministry of Health office, WHO regional office in Africa and the Rotavirus Regional Reference Laboratory at Sefako Makgatho Health Sciences University in South Africa for their support.
The current study was supported by grants from the GAVI alliance through WHO - AFRO rotavirus surveillance program through the Ministry of health and Kenya Medical Research Institute.