Background: A sport-related concussion (SRC) is a mild traumatic brain injury resulting from impulsive forces in sports, often presenting various symptoms. Clinicians lack objective measures and rely on subjective patient reports (via symptom checklists or inventories) to guide return-to-play decisions. These methods, however, may not reflect physiologic recovery, with concern over the under-reporting of symptoms to return to activity quicker. Quantitative tracking of physiologic recovery could offer a more personalized approach to treating concussion. Following concussion, neurometabolic events unfold impacting neuronic membranes and disrupting ionic balance. Sodium-potassium pumps increase, boosting ATP production but depleting glucose as well leading to an inability to utilize glucose effectively. During this period alternative energy substrates may be used potentially including ketone bodies. Acetone, a ketone body that is produced when the main ketone body is broken down to be used for energy, is small and able to be exhaled in the breath and measured non-invasively. Tracking the altered metabolism and understanding how those changes affect symptomology could offer a quantitative measure useful for clinicians. This study, part of the BrACKS initiative, aims to explore breath acetone as a marker for concussion recovery, assessing its association with symptom severity and number.
Methods: In this cross-sectional study six collegiate athletes from a single Division I university including (N = 5) football players, and (N = 1) soccer player diagnosed with a SRC during their competitive season were recruited for the study. Breath acetone was measured in parts per million by portable breathalyzer and symptoms measured by the Sport Concussion Assessment Tool (SCAT-5) daily until participants were 14 days post-concussion or cleared to return to activity. Statistical assessments evaluating the dependency of breath acetone on symptom measures, exploring the relationship between daily changes in breath acetone and symptom measures, and determining how larger or smaller changes in symptoms affect breath acetone.
Results: Breath acetone exhibited individualized patterns like symptom severity and number. No significant association was found between breath acetone and symptom severity or number, but a significant associative relationship was observed in daily changes between breath acetone and symptom number.
Conclusion: While breath acetone did not show a direct association with symptom burden, the study suggests potential for quantitative biomarkers to complement traditional symptom tracking. Further longitudinal research with larger cohorts is essential for understanding this relationship and making recommendations.
Breath acetone, Ketone, Neurometabolic cascade, Traumatic brain injury
SRC: Sport-Related Concussion; ATP: Adenosine Triphosphate; BrACKS: Breath Acetone Concussion Ketone Study; SCAT: Sport Concussion Assessment Tool; PPM: Parts per Million; IFN-γ: Interferon Gamma; SCOAT: Sport Concussion Office Assessment Tool
A sport-related concussion (SRC) is a traumatic brain injury occurring in sport because of impulsive forces being transmitted to the brain due to a direct blow to the head, neck, or body that present with a myriad of symptoms following injury [1]. With the absence of objective evaluation measures and structural abnormalities seen on neuroimaging, clinicians must rely on a patient’s subjective symptom reporting following SRC (often through the use of symptom reporting checklists or inventories) to decide when to begin a return to activity and sport. Symptoms of a SRC are expected to resolve in 10-14 days and many clinical management protocols center on that timeline [2,3]. Although the subjective symptom checklists are widely used, the American Medical Society for Sports Medicine notes that current methods of evaluation and monitoring may not reflect physiologic recovery [4]. Additionally, while many clinicians feel confident about managing SRC, there is concern about the accuracy of player-reported symptoms as players may under-report to return earlier to sport [5]. Tracking physiologic recovery quantitatively may offer a less subjective and more personalized method of evaluation and management of SRC.
Following a concussive injury, a series of neurometabolic physiologic events unfolds. The mechanical impact stretches neuronic membranes, causing the release of neurotransmitters and disrupting the equilibrium of ions [2]. To restore homeostasis, sodium-potassium pumps increase activity, leading to heightened ATP production and the utilization of glucose as an energy substrate [2]. The acute overuse of glucose to address the energy crisis depletes circulating glucose and glycogen stores resulting in a glucose depression (which varies with age and injury severity) and a prolonged period of diminished cerebral glucose metabolism [2,6]. The increased utilization of sodium potassium pumps also prompts extensive calcium accumulation in the mitochondria, causing a reduction in oxidative metabolism that typically lasts for 7-14 days [6,7]. During this period, alternative substrates, such as ketone bodies, come into play to provide energy. Ketone bodies, derived from metabolism of free fatty acids in the liver, offer an alternative option when glucose is unavailable to be used. The primary ketone body, acetoacetate, can be enzymatically converted to its other forms, β-hydroxybutyrate for energy, and acetone which is released as breath [8]. The use and subsequent conversion of acetoacetate into β-hydroxybutyrate and acetone presents a potential measurement point.
In addition to providing an alternative source of energy, ketone bodies also have therapeutic potential. Utilization of ketones can improve cell survival, reduce oxidative stress, regulate inflammation, and reduce neurodegeneration in brain injuries [9]. They may also improve global and regional cerebral blood flow which has previously been linked to symptom resolution time [9,10]. Breath acetone has been established as a potential biomarker for its prognostic, diagnostic, and therapeutic monitoring capabilities of changes in metabolism in other pathologies such as heart failure, dietary ketoacidosis, Alzheimer’s disease, Parkinson’s disease, dementia, cancer, type 2 diabetes, and epilepsy [8,9,11,12]. There are no known studies directly evaluating how breath acetone responds following concussion. However, there has been a recent feasibility study where researchers provided a ketogenic diet and medium-chain triglyceride supplementation intervention to individuals who had sustained a traumatic brain injury and suffered post-concussion syndrome eliciting improvements in both symptoms and visual memory [13]. In healthy individuals not fasting or consuming a low-carbohydrate diet, breath acetone is typically measured at a concentration of 0-2.00 ppm and levels higher than that could indicate disease or an acute condition [14].
The present investigation is part of a larger proof of concept study exploring the response and potential of breath acetone as a clinical marker of concussion recovery in the Breath Acetone Concussion Ketone Study (BrACKS). Currently, there is no available evidence exploring the role of breath acetone in sports-related concussion and limited studies assessing the impact metabolic changes have on this injury. This investigation aims to assess if current methods of tracking recovery post-concussion are related to the neurometabolic changes that occur as measured by breath acetone. As physiologic resolution of disrupted oxidative metabolism and resolution of symptoms are similar in time span, it is possible a relationship exists between these methods of testing. It is hypothesized that there is an association between breath acetone and symptom burden in both severity and number during concussion recovery.
Division I football and soccer athletes from a single institution were recruited during their Fall 2021 competitive seasons once diagnosed with an SRC by a team physician and Athletic Trainer. Upon diagnosis, participants met with research staff to assess if they met the inclusion criteria or exhibited exclusion factors. For inclusion in the study, participants had to be active student-athletes in football or soccer, aged 18-26 years-old, and experience an SRC within two weeks from potential enrollment. In total, 8 athletes sustained an SRC during the competitive season, which was approximately 5.71% of the 140 athletes monitored. Potential participants were excluded if they had an underlying medical condition known to impact the testing protocol or breath acetone values (e.g., diabetes, asthma, chronic neurological disorder, etc.), or simply could not withstand the testing protocol of daily breath assessments. Additional exclusionary criteria consisted of practicing a low-carbohydrate or ketogenic diet as this would affect concentration of breath acetone. One athlete did not meet inclusion criteria due to age, and another athlete withdrew consent on the second day of testing due to testing burden. The included participants were mainly football players (N = 5), though there was one soccer player (N = 1). Concussions from these participants occurred throughout the competitive season during both practice and game play. Their demographics are displayed within Table 1.
Table 1: Participant demographics. View Table 1
The association of breath acetone and symptom burden was evaluated through a cross-sectional study design with emphasis as to not overly burden the athletes and to provide descriptive measures without intervention. Following concussion diagnosis and study enrollment, athletes were measured daily with a breath acetone breathalyzer and the Sport Concussion Assessment Tool 5 (SCAT-5) symptom evaluation checklist [15]. The symptom evaluation measurement was always completed prior to obtaining breath acetone concentration and used as a wash period. Both were assessed with the athlete in a seated position. In total, the daily testing lasted no more than 15 minutes (Figure 1). Measurements were taken when athletes were evaluated by athletic trainers for their SCAT-5 symptom scores which varied based on their daily schedule. Daily measurements began after diagnosis and enrollment was complete (as early as the day after concussion for two participants), and were continued until the participant was cleared, or until 14 days post-concussion Three participants were cleared to return to sport at -9, -10, and -14 days, respectively. For comparative analysis purposes of acute response, only measurements of the first 14 days were used for evaluation. Baseline (non-concussed measurements prior to the start of the season) SCAT-5 symptom scores are taken as part of standard sports medicine procedure and were sourced for each concussed participant to establish their own normative value. The present study was approved by Institutional Review Board #300005418 and informed consent obtained.
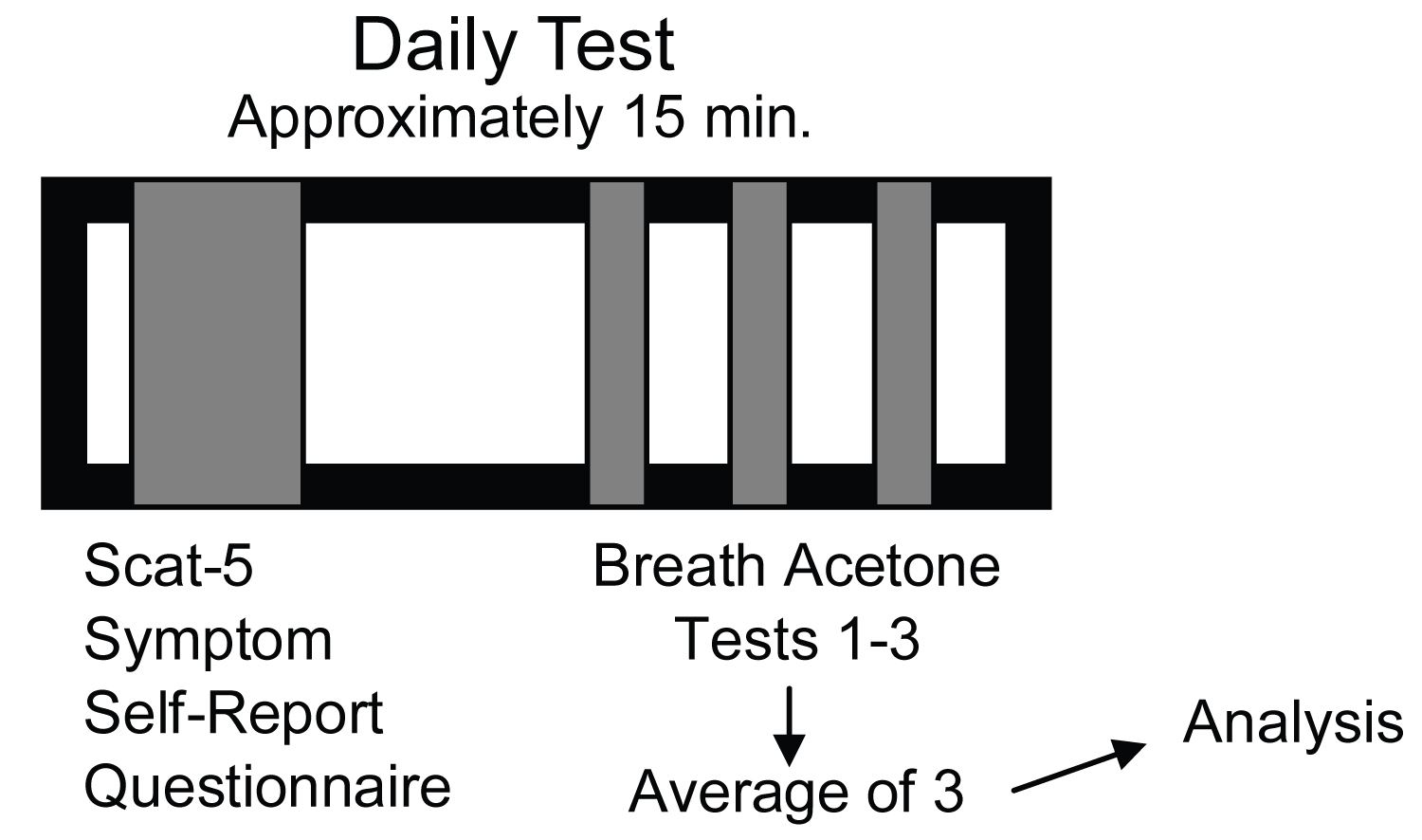 Figure 1: Diagram of daily post-concussion testing procedures.
View Figure 1
Figure 1: Diagram of daily post-concussion testing procedures.
View Figure 1
Breath acetone was assessed daily following concussion diagnosis and enrollment in the study with a portable breathalyzer (Ketonix ® Professional, Ketonix ® , Stockholm, Sweden), able to detect acetone concentration at a range of 0-200 ppm (parts per million). Participants followed device directions to maintain methodology and a tidal breath technique (approx. 8 seconds) was used for each measure because of its repeatability [16]. Daily breath acetone was obtained by taking three measures consecutively and then assessing the means to generate a daily value, thus increasing statistical power.
Symptom burden was measured with the SCAT- 5 symptom evaluation list [15]. Participants rated their symptoms for 22 items on a 7-point scale, consisting of none (0), mild (1-2), moderate (3-4), or severe (5-6). Measures consisted of the total number of symptoms (out of 22) and the symptom severity, which was a total sum of all symptoms (out of 132). A higher total number of symptoms and severity indicated greater symptom burden.
The software, XLSTAT v. 3.1 (Data Analysis and Statistical Solution for Microsoft Excel, Addinsoft, Paris, France 2022), was used as the primary statistical software for this investigation of both descriptive statistics and hypothesis tests. Tests were completed across 14 days following concussion to account for early metabolic changes as the response of breath acetone is not well-understood. Assessments for trends in individuals’ breath acetone concentration and symptom burden (severity and number) over the 14 days post-concussion were completed with a non-parametric Mann-Kendall Trend test to verify visual inferences as the data was not normally distributed as determined by Shapiro-Wilk Tests (Symptom Number W = 0.876, P < 0.0001; Symptom Severity W = 0.723, P < 0.0001; Breath Acetone W = 0.0731, P < 0.0001). A Kruskal-Wallis test was used to differentiate between individual participant’s values of breath acetone and symptom burden, evaluating the uniqueness of everyone’s results. Kendall’s Tau-B method of testing dependency was completed to determine association between symptom burden and breath acetone due to lack of variable normality and small sample size. To assess whether changes in breath acetone between days were related to changes in symptom burden between days, we created categorical variables denoting whether the variable was higher, lower, or the same from their previous day measurements and a Fischer’s Exact Test was employed due to the sample size. Categorical variables were created to compare those that had either a minor change (less than sample mean) or a substantial change (greater than sample mean) in their symptom severity or symptom numbers from their baseline values to their highest measured value in the first 14 days post-concussion. Comparisons of breath acetone to these changes from baseline were completed utilizing Mann-Whitney tests for non-parametric data. Significance for all statistical tests was established as P < 0.05.
Breath acetone measurements during the first two weeks post-concussion were unique depending on individual and were absent of a visual trend (Figure 2). One participant, Participant 4, did however, exhibit a slight descending trend in breath acetone that was statistically significant (Table 2). Conversely, measurements of symptom burden (severity and number) demonstrated descending trends both visually and statistically in all participants except the Participant 4 in the determinant of symptom number where though there was a descending trend, it was not statistically significant (Figure 3a, Figure 3b and Table 2). In the first 14 days post-concussion breath acetone was not statistically dependent with symptom severity (r τ = -0.050, P = 0.594) or symptom number (r τ = -0.015, P = 0.874). Symptom burden (severity and number) demonstrated significant interdependency with each other at both timepoint assessments (r τ = 0.949, P < 0.0001).
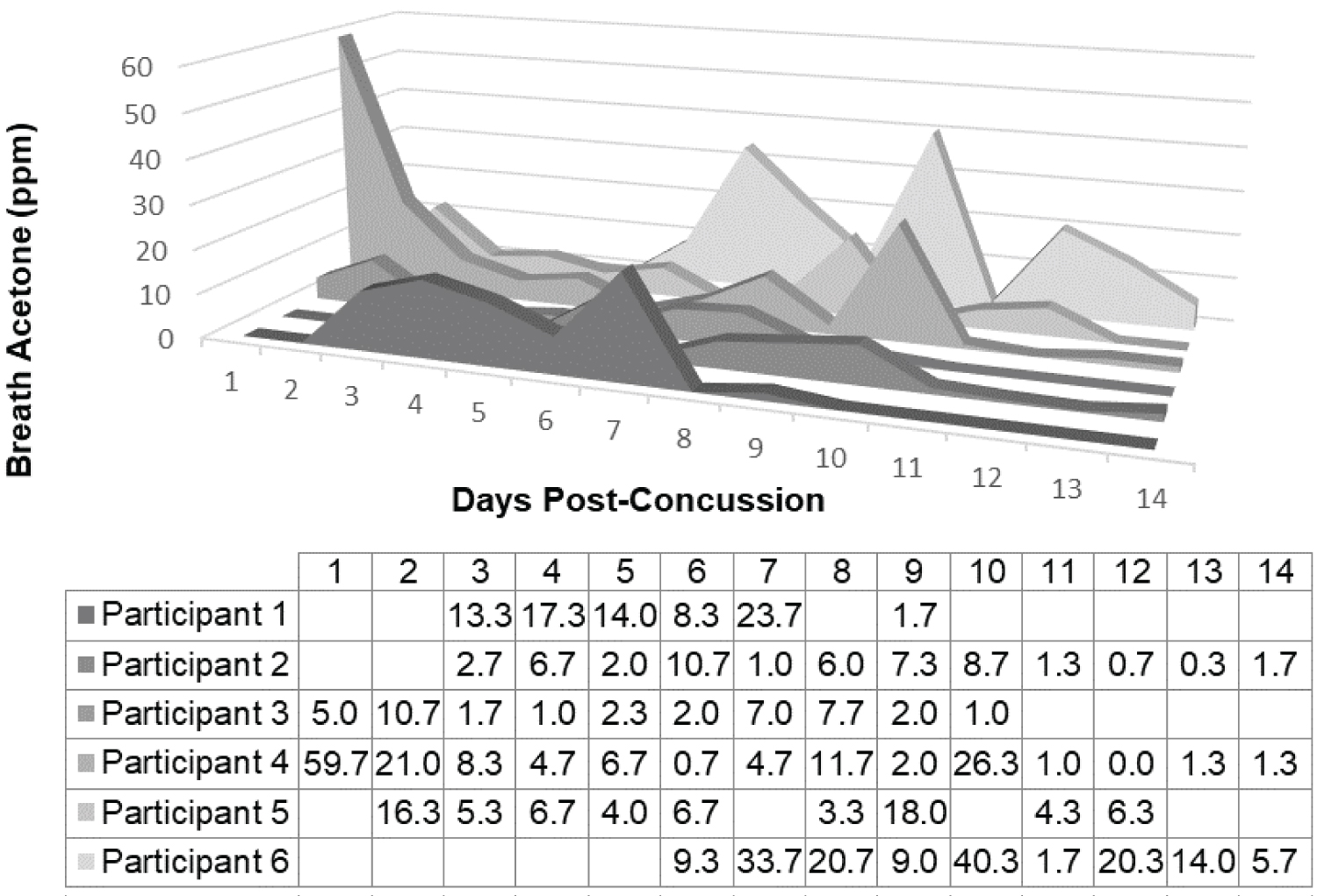 Figure 2: Breath acetone (ppm) measurements across the first 14 days post-concussion for each participant (N = 6). Days post-concussion start on the first day after concussion as no participants were measured on the day of concussive injury. During the first 14 days following concussion breath acetone was measured between 0-59.67 ppm (6.33 ± 9.17; Median ± IQR). The results of participants were deemed distinct based on Kruskal-Wallis test (P = 0.014).
View Figure 2
Figure 2: Breath acetone (ppm) measurements across the first 14 days post-concussion for each participant (N = 6). Days post-concussion start on the first day after concussion as no participants were measured on the day of concussive injury. During the first 14 days following concussion breath acetone was measured between 0-59.67 ppm (6.33 ± 9.17; Median ± IQR). The results of participants were deemed distinct based on Kruskal-Wallis test (P = 0.014).
View Figure 2
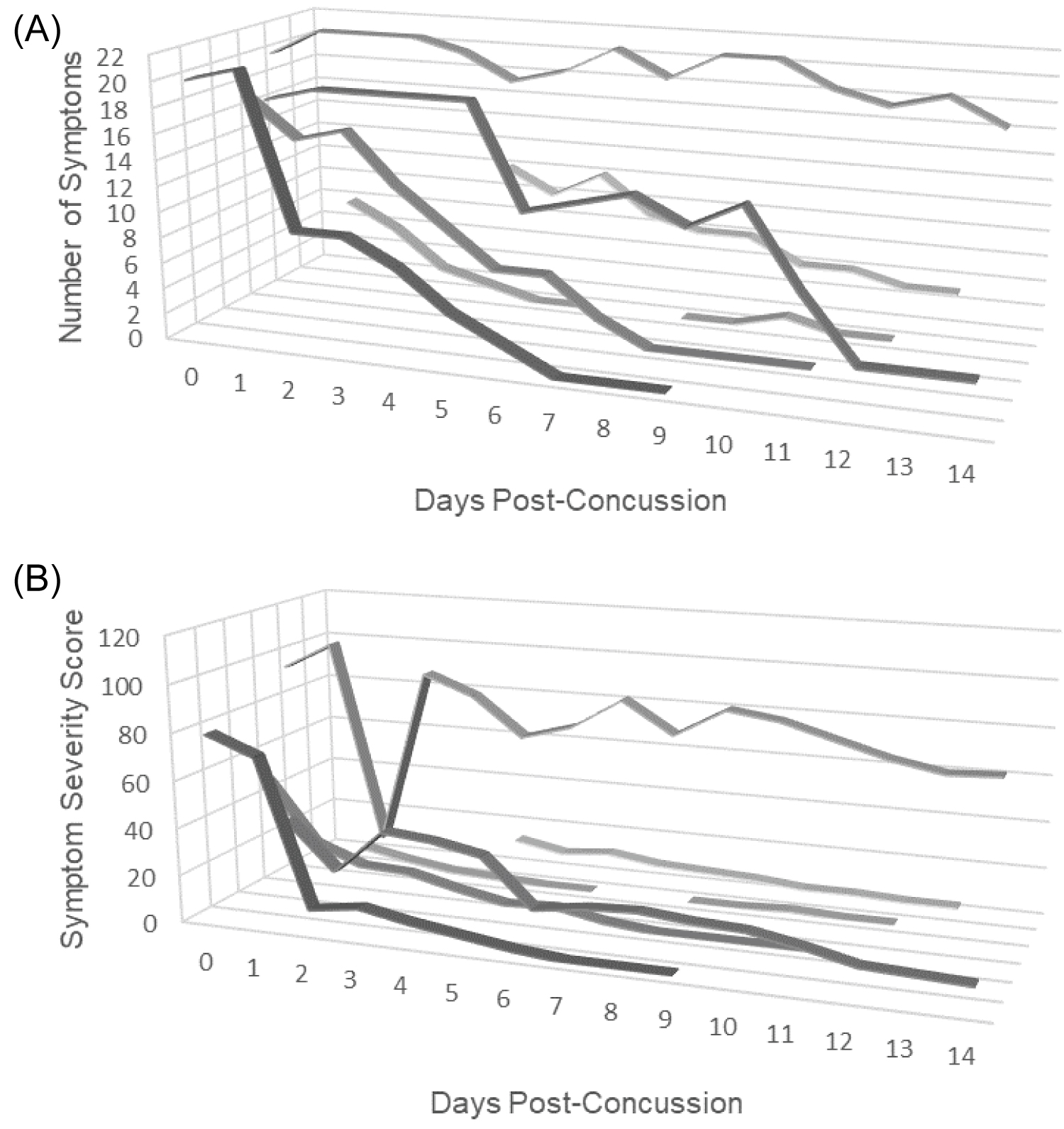 Figure 3: (A) Symptom number measurements across the study period for each participant (N = 6). Number of symptoms ranged from 0 to 22 out of 22 (7 ± 17; Median ± IQR). Participant measures in the first 14 days were unique by individual (P < 0.0001); (B) Symptom severity score measurements across the study period for each participant (N = 6). During the study period, daily symptom severity measurements ranged from 0 to 106 out of 132 (9 ± 32; Median ± IQR). Participant measures in the first 14 days were unique by individual (P < 0.0001).
View Figure 3
Figure 3: (A) Symptom number measurements across the study period for each participant (N = 6). Number of symptoms ranged from 0 to 22 out of 22 (7 ± 17; Median ± IQR). Participant measures in the first 14 days were unique by individual (P < 0.0001); (B) Symptom severity score measurements across the study period for each participant (N = 6). During the study period, daily symptom severity measurements ranged from 0 to 106 out of 132 (9 ± 32; Median ± IQR). Participant measures in the first 14 days were unique by individual (P < 0.0001).
View Figure 3
Table 2: Mann-Kendall Trend test statistics evaluating the presence of trends in breath acetone and symptom burden across time for each participant throughout the study. View Table 2
The two-tailed Fischer’s Exact Test determined there were significant associations between daily changes in breath acetone and daily changes in symptom number (P = 0.069) within 14 days post-concussion. When symptom numbers were higher than the day before, there was an association with breath acetone being higher than the day before as well (P = 0.034). There was, however, no significant association between the changes in symptom severity and breath acetone, or other changes in symptom number (lower or same measures from the day before).
Five of the six participants had their baseline (preseason, non-concussed) symptom characteristics available. In the sourced baseline SCAT-5 assessments, the average symptom severity score amongst participants was 1.2 out of 132 and the average symptom number in participants was 0.8 out of 22. When comparing symptom burden reported on the first day of concussion to baseline (non-concussed) values, all participants demonstrated higher numbers of symptoms and severity with the number of reported symptoms averaging 15.6 higher than baseline, and the severity ratings averaging 48.4 ratings higher than baseline. There were significant differences in breath acetone between participants with a larger or smaller change in values from baseline when measures were assessed via Mann-Whitney Test. Statistically significant differences between groups were found when symptom number was evaluated (U = 167.5, P = 0.023), but not when symptom severity was evaluated (U = 264, P = 0.254).
The central hypothesis of this investigation is there being an association between breath acetone and symptom burden (severity and number) during concussion recovery. There was no statistical association determined between breath acetone and symptom burden within the first 14 days post-concussion. However, several inferences are present within the results. In the first two weeks of concussion, when the number of symptoms rose from the previous day it was statistically likely that breath acetone would also be higher. Additionally, there was a statistically significant difference between breath acetone of participants whose difference between their highest number of symptoms in the first 14 days post-concussion and their baseline number of symptoms was higher or lower than the sample mean.
Researchers have suggested clinical symptoms may be related to physiological events of the neurometabolic cascade [17], but none have been definitively proven other than the time similarities in resolve of symptoms and biometrics such as cerebral blood flow [10]. Estimates of inflammatory markers have been suggested to correlate to symptom severity, though these markers contradicted themselves by gender with a high interferon gamma (IFN-γ) in males being associated, but a lower IFN-γ in females being associated [18]. It is unclear if the method of subjective self-surveying accurately details present symptoms. Though reliability and validity of the method of using the SCAT tests have been proven [19], the results of this study may indeed echo the message from the American Medical Society for Sports Medicine that current methods potentially do not reflect physiologic recovery from concussion accurately [4]. Indeed, they may be measuring different aspects of the injury process and have no clinical or statistical correlation or dependency in which case multiple methods of testing may be required (both symptom-based and an objective biomarker). Recommendations currently exist to incorporate two or more rapid, non-invasive, sideline tests into the concussion diagnostic process [19]. The same recommendations may be warranted for the recovery process to provide subjective and objective measures for clinical decision making.
Our results from this study demonstrated relationships between the number of symptoms reported and breath acetone. This was seen in the association that occurred between the rise of symptom numbers and the rise of breath acetone within the first 14 days. It was also visible in the significant differences of breath acetone between groups stratified as those having higher or lower difference than the sample mean when their highest 14-day value was compared to their baseline. Symptom severity did not elicit the same relationships, despite there being such an extensive dependency between the two variables. This could be due to the subjective self-reporting of symptom numbers. It is reasonable to assume that it is easier for a concussed individual to gauge the presence of a symptom rather than gauge the severity of that symptom. Indeed, while the reporting of the presence of a symptom may be similar in individuals, an individual’s perception of severity may be wildly different between individuals. These differences in reporting are likely due to a variety of factors. Attempts have been made to address these subjective reporting biases. Following the release of the 6 th International Consensus Statement on Concussion in Sport, the development of the Sport Concussion Office Assessment Tool 6 (SCOAT6) established clinical evaluation of SRC 72-hours post-injury. Variations from the SCOAT6 to SCAT-6 consisted of added items to assess autonomic dysfunction and global symptom scales. Specifically, on the symptom checklist, the SCOAT6 queries concussed athletes about abnormal heart rate and excessive sweating, along with an orthostatic vital sign assessment looking at blood pressure, heart rate, and symptoms related to orthostatic intolerance (e.g., dizziness, fainting, blurry/fading vision).
In this investigation that focused on the first 14 days of post-concussion recovery, trends were seen both visually and statistically. Individuals had statistically different breath acetone and symptom burden over their recovery. This is an important finding to advocate for the potential to personalize treatments using the sensitivity of the biomarker. Visually, individuals exhibited similar decreasing trends in symptom burden (both severity and number) over the first two weeks. Whereas, in terms of breath acetone there seemed to be differences between individuals in where they peaked. All but one participant exhibited the trends in symptom burden (Participant 4 not demonstrating a trend in number but only severity). That same participant was the one that demonstrated a decreasing trend in breath acetone over the 14 days. Though this occurred in one participant there is a possibility of an inverse relationship between breath acetone and symptom burden. Inverse relationships should be considered statistically with future research including large sample sizes. The participant in question was a participant that was not cleared during the study period to return to activity. The presence of an inverse statistical relationship could thus potentially allude to prognosis and recovery time. Further research with more participants is required to understand prognostic information because of the small sample size and early-stage nature of this novel investigation. The nature of this investigation was unique in that it mirrored the daily monitoring of athletes’ post-concussion outcomes that is currently occurring in many universities. Though one participant dropped out from the study due to burden, the individual had never had a concussion and was unaware that daily monitoring was the norm at a Division I institution. The overall burden of the breath testing was low, and the investigation did not impact the clinicians’ decisions on return to activity. Bowing to the desire to keep testing burden low and provide an observational frame of research, daily testing was not mandated to occur at a specific time of day and there was variation in the time of testing. The time of day that testing occurred was evaluated for its impact on breath acetone through exploratory analyses and was found to not have a significant impact on the measures. Participants were asked to not consume food or beverages within 30 minutes of their test to ensure nutrient intake did not impact measurements. Symptom monitoring and breath acetone measurements were not taken after the athlete was returned to activity to provide a more longitudinal view of physiologic recovery, which is a limitation to this study. In future iterations testing after the participant has returned to activity would provide insight on long-term physiologic recovery and prognostic potential.
The small sample size was a limitation to this study and limited the statistical tests that could assess the primary aims. For instance, though differences were noted when categorically comparing those with higher or lower symptom number changes from their baseline symptom scores, the U values were notably high suggesting the results could have an element of chance. This was an expected limitation, however, in that it was completed at a single site and the number of concussions that occurred (approximately 5%) were like that occurring within Division I football players during a season [20]. To make any clinical recommendations or generalizations one would need to repeat this study with a larger cohort, particularly with multiple sites, to increase statistical power.
In this investigation, breath acetone was not significantly associated with symptom number or severity during concussion recovery within the first two weeks post-concussion. However, there was an associative relationship between symptom number and breath acetone in terms of changes between days, as well as a significant difference in breath acetone when assessing higher and lower symptom number changes from baseline. The current methodology of symptom tracking has been criticized by the public for its reliance on subjective reporting. The association between changes in number of symptoms and breath acetone suggests there could be a relationship. Further research that is longitudinal and utilizes a larger cohort is needed to understand the nature of changes in breath acetone and how those changes relate to symptom burden. Symptom tracking remains the standard for monitoring concussion recovery, however, this research suggests there is potential for quantitative biomarkers to provide additional decision-making information to clinicians.
We would like to acknowledge Kent and Maxine Falb of the Professional Football Athletic Trainers’ Society for their funding of this research grant through the National Athletic Trainers’ Association Research and Education Foundation.
The work of this investigation was supported by the National Athletic Trainers’ Association Research and Education Foundation Doctoral Grant #1920DGP05. The authors declare no other conflicting interests in this research.
The authors named in this manuscript contributed equally to this work.