AD: Alzheimer Disease; AIS: Acute Ischemic Stroke; ASD: Autism Spectrum Disorder; BBB: Blood Brain Barrier; BC: Breast Cancer; BD: Bipolar Disease; CCI: Controlled Cortical Impact; CM: Cerebral Malaria; cMLCK: Cardiac Myosin Light Chain Kinase; CNS: Central Nervous System; CRD: Cysteine-rich Domain; CSC: Cancer Stem Cell; ECM: Experimental Cerebral Malaria; EGF: Epidermal Growth Factor; hCMEC: Human Brain Microvascular Endothelial Cell; HIV: Human Immunodeficiency Virus; IG: Immunoglobulin; IL: Interleukin; iPSC: Induced Pluripotent Stem Cell; iRBC: Infected Red Blood Cell; JAG1: Jagged 1; KDa: Kilo-Daltons; LOAD: Late Onset Alzheimer's Dementia; MAP: Mitogen-activated protein; ND: Neuropsychiatric Disorders; NMDA: N-methyl-D-aspartate; NRG: Neuregulin; NTAK: Neural and Thymus-Derived Activator for ErbB Kinases; OGD: Oxygen Glucose Depravation; OPC: Oligodendrocyte Progenitor Cells; PI3-K: Phosphatidyl-Inositol-3-Kinase; PIMs: Pro-inflammatory Markers; pMCAO: Permanent Middle Cerebral Artery Occlusion; RBC: Red Blood Cell; RNA: Ribose Nucleic Acid; SERCA2a: Sarco/Endoplasmic Reticulum Ca2+ ATPase 2a; SG: Stress Granules; STAT3: Signal Transducer and Activator of Transcription 3; SZ: Schizophrenia; TBI: Traumatic Brain Injury; USD: United States Dollar
Neuregulins, a family of EGF-like signaling molecules, are involved in cell-cell crosstalk and play an important role in development, maintenance and repair of the nervous system, heart, breast and other organs. Independent studies described a ligand for the oncogene ErbB2 (neu, Her2) and factors that stimulated proliferation of Schwann cells, as well as synthesis of receptors for acetylcholine by muscle. These ligands and factors are essentially products of the same gene, referred to by Marchionni M. as neuregulin (NRG-1) [1]. Besides NRG-1 gene, there are other genes that encode related proteins, such as NRG-2 (Don-1, NTAK), NRG-3 and NRG-4. There are few studies that describe NRG-2, -3 and -4 and a complete analysis of their function remains a challenge. However, NRG-2 was recently reported as a component of stress granules (SG), microscopically visible aggregates of translationally stalled messenger ribonucleoprotein complexes that are formed in response to direct stress conditions [2]. Furthermore, it was shown that NRG-2, secreted from astrocytes, bound to ErbB3 on neurons and promoted neuronal survival [3]. NRG-3 was shown to be associated with schizophrenia (SZ) in a Chinese population [4], with bipolar disorder (BD) [5] and with the risk and age of onset of Alzheimer disease (AD) [6]. In addition, NRG-3 has a key function in promoting early mammary morphogenesis and is involved in breast cancer (BC) [7]. NRG-4 expression was decreased in human inflammatory bowel disease samples and mouse models of colitis, suggesting that activation of ErbB4 is altered [8]. An interesting study [9] showed that NRG-4 overexpression prevents high fat diet-induced weight gain and fatty liver and reduces obesity-induced chronic inflammation.
Considering the increasing interest and research focused on NRG-1 in the past years, this review summarizes what is currently known about NRG-1 and its impact on health and disease as well as its current and potential use(s) as a CNS anti-inflammatory agent against inducers of brain inflammation and injury as well as in the treatment of various neurological disorders.
NRG-1 gene encodes 21 exons located on human chromosome 8p22-21. Alternative splicing of more than 30 exons in different parts of the NRG-1 transcript generates more than 30 isoforms that can be grouped into six types (I-VI) [10-12] (Figure 1). They are synthesized as transmembrane precursors consisting of either an immunoglobulin-like (Ig) domain or cysteine-rich domain (CRD), an EGF-like domain, a transmembrane domain and a cytoplasmic tail [13]. NRG-1 types I and II are released from the cell surface after protease-mediated proteolytic cleavage and may function as paracrine signals [14]. NRG-1 type III remains tethered to the cell membrane after cleavage and acts as a juxtacrine signal [15]. Full-length type IV spans 1.8 kb and encodes a putative protein of 590 amino acids with a predicted molecular mass of approximately 66 kDa [15]. Furthermore, there are two major classes of NRG-1 identified as α and β isoforms. NRG-1α and NRG-1β contain related, but structurally distinct EGF-like domains composed of a common amino terminal segment followed by α or β variant sequences [16]. NRG-1β isoforms predominate in the nervous system while α isoforms are common in mesenchymal cells and are critically important for breast development [14]. Type I NRGα and β isoforms are the predominant isoforms expressed in early embryogenesis, whereas type II and type III are undetectable until the mid-gestation stage [17]. Type I or Heregulin is an acetylcholine receptor activator, type II is a Glial Growth Factor, type III is a sensory and motor neuron-derived factor and type IV is involved in neuronal activity regulation. Functions of types V and VI are not well known and their investigation remains a challenge. Types I, II and III of NRG-1 express in human peripheral blood in addition to neurons, while types IV and V express specifically in the brain [18].
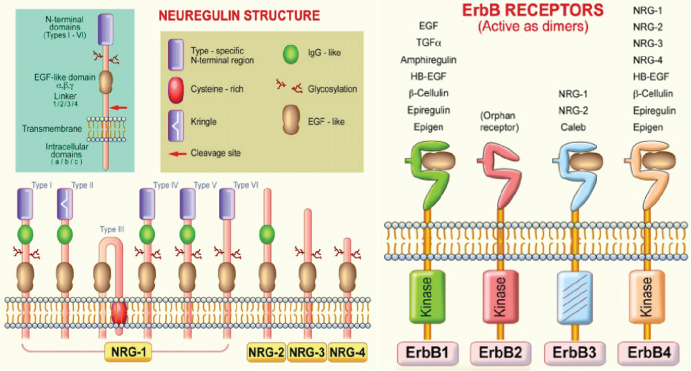 Figure 1: Neuregulin (NRG) and ErbB receptor structures. NRG gene products share a structural characteristic for the extracellular epidermal growth factor (EGF) domain and it differentiates this subfamily from other members of the EGF family. All EGF family members are ligands of ErbB receptors, although with varying affinities, with ErbB3 and ErbB4 being specific NRG-binding receptors. NRG-1 isoforms have been classified in types I-VI on the basis of differences in the NH2-terminal distal region [19].
View Figure 1
Figure 1: Neuregulin (NRG) and ErbB receptor structures. NRG gene products share a structural characteristic for the extracellular epidermal growth factor (EGF) domain and it differentiates this subfamily from other members of the EGF family. All EGF family members are ligands of ErbB receptors, although with varying affinities, with ErbB3 and ErbB4 being specific NRG-binding receptors. NRG-1 isoforms have been classified in types I-VI on the basis of differences in the NH2-terminal distal region [19].
View Figure 1
Neuregulins transmit their signals to target cells by interacting with transmembrane tyrosine kinase receptors of the ErbB (HER) family (Figure 1) [19]. This family includes four members of the epidermal growth factor receptor (EGF-R, ErbB1, ErbB2, ErbB3, and ErbB4). Receptor-ligand interaction induces the heterodimerization of receptor monomers, which in turn results in the activation of intracellular signaling cascades and the induction of cellular responses. In vivo, functional NRG-1 receptors are heterodimers composed of ErbB2 with either an ErbB3, or ErbB4 molecule [20]. This interaction activates various signaling pathways including the mitogen-activated protein (MAP) kinases and phosphatidyl-inositol-3-kinase PI3-K/Akt survival pathways [21,22]. NRG-1 effects are associated with the regulation of both canonical and alternative NF-kB signaling pathways [23]. Increased cardiac mechanical stress leads to upregulation of NRG-1. Interestingly, integrins, a family of receptors expressed on cardiomyocytes, act as sensors for mechanical stress and can non-canonically bind NRG-1 [24]. NRG-1 regulates cell maintenance, differentiation, proliferation, migration, and survival or apoptosis in neuronal and non-neuronal cell types [11].
Many cell types express NRG-1, among those are microglial and neuronal cells, in response to several stimuli such as tissue injury, blood brain barrier (BBB) dysfunction and neuronal cell damage [25]. NRG-1 has anti-inflammatory properties. For example, recombinant human NRG-1 decreases the production of superoxide and nitrite by stimulating mouse brain N9 microglial cells [26]. Furthermore, NRG-1 treatment in a cerebral ischemia model induced a decrease in microglial activation and IL-1 mRNA expression. NRG-1 reduced the expression of inflammatory genes associated with leukocyte migration and activation [27]. In contrast, early NRG-1 mutations induced defects in the developing nervous system and embryos died in utero due to cardiovascular failure.
In Drosophila central nervous system (CNS), neuronal-glia interactions are necessary for the formation of the longitudinal axon trajectories. The protein Vein, found in drosophila, is similar in structure to NRG and is produced by neurons and leads to the survival of glia [28]. In Caenorhabditis elegans, a simple version of the NRG-ErbB is found, with a single receptor, the product of the let-23 gene and a single ligand encoded by lin-3 [1]. An interesting study developed in fish showed that NRG-1 is found in the axolotl (Ambystoma mexicanum) peripheral nervous system and blastema and is capable of regeneration of amputated limbs, to the point of digit formation [29]. In a zebrafish model, which is hypoxia tolerant, NRG-1 plays a role in establishing the cardiac nervous plexus, while inadequate innervation leads to deficits in cardiac maturation, function and survival [30]. Although flies, worms and fish have been used to understand the functions of simple conserved signaling systems, like the EGF/ErbB pathway, studies in invertebrates have been limited and challenging. Analysis of functions are limited due to the diversification of their corresponding ligands and receptor molecules, unlike in higher organisms [31]. NRG-1 is highly expressed in the developing brain and remains expressed in the adult nervous system [32]. Studies in heterozygous NRG-1 knockout mice showed that the animals exhibit impairments in prepulse inhibition and working memory [33]. However, an interesting study using transgenic mice overexpressing NRG-1 showed that aberrant hyper-signals of NRG-1 also disrupt various cognitive and behavioral processes, but further experiments are necessary to clarify these observations [34]. In a rat model, hypothalamic NRG-1 was transported to the posterior pituitary as the β form. Thus, NRG-1β may function via the regulation of prolactin expression through a paracrine mechanism [35-38]. In addition, a NRG-1 study in non-human primate rhesus monkey assessed the differential expression of NRG-1 and its ErbB receptors in gastrointestinal structures, and found higher expression levels in stomach and small intestine [35]. All these models assessed the complex involvement of NRG-1 in development and in adult life, although many aspects need further investigation.
During embryogenesis, NRG-1 expression leads to cardiomyocyte proliferation. Studies found that there is an interaction between hyaluronan and Erb signaling during endocardium development [39]. NRG and Notch1 are necessary for the development of the atrioventricular conduction system [40]. In the adult heart, NRG-1 is located in endothelial cells, playing an important role in regulation of survival, hypertrophy, proliferation and interaction between cardiomyocytes [41]. Furthermore, NRG-1 provides protection against apoptosis, myofibrillar disarray, anthracycline-induced cardiomyopathy, and scar formation [42]. These data underline the importance of NRG-1 in cardiovascular development, as well as in cardiac maintenance during the adult life.
Many studies have shown the protective effects of NRG-1 in heart disease. Administration of NRG-1 improved cardiac function via SERCA2a (Sarco/Endoplasmic Reticulum Ca2+ ATPase 2a) and cMLCK (Cardiac Myosin Light Chain Kinase) in a rat heart failure model [43] and significantly enhanced cardiac function in patients with chronic heart failure [44]. Furthermore, NRG-1β treatment leads to iPSC (induced pluripotent cell) differentiation into ventricular-like cardiac cells, which are capable of preserving cardiac function and tissue viability when transplanted in a mouse model of acute myocardial infarction [45]. NRG-1 and ErbB receptors are expressed in ex vivo skeletal muscle vascular endothelial cells; the addition of NRG-1 induces angiogenesis [46]. Furthermore, elevated expression of NRG‑1 increased the number of microvessels formed in the ischemic myocardium [47]. Although NRG-1 plays an important role in cardiac development and in tissue recovery following heart ischemia, potentially including it in adjunctive therapy in the treatment of cardiac diseases remains a challenge.
In head and neck cancer, tumor cells control the amount of cell-surface NRG-1 available for cleavage and ErbB3 activation [48]. Constitutively active forms of HER/ErbB receptors have been reported in tumors and their activation may occur through their overexpression in the plasma membrane or through their structural alterations [49]. In the invasive mucinous adenocarcinoma of the lung, molecular lesions of the NRG-1 gene leads to aberrant activation of ErbB2/ErbB3 signaling [50]. NRG-1 treatment induced cancer stem cell (CSC) characteristics in breast cancer (BC) cell lines in vitro [51]. Furthermore, NRG-1 produced by BC cells can enhance transendothelial migration and intravasation, due to induction of jagged-1 (JAG1), an important component of the Notch pathway [52]. In BC, NRG-1-dependent activation of HER3 induces primary resistance to trastuzumab in HER2- [53] overexpressing breast cancer cells [54]. In gastric tumors, fibroblasts secreted NRG-1, which in turn increased the stem cells self-renewal and its overexpression was correlated with gastric cancer prognosis [55]. NRG-1 was demonstrated to be a prominent growth factor during cultivation of spheroids in vitro from non-small cell lung cancer specimens and pleural effusion [53]. Another study showed that elevated levels of ErbB3 on ovarian cancer cells and corresponding ligand NRG-1 in the omentum allowed for tumor cell metastasis and growth in the omentum [56]. Although it has been demonstrated that NRG-1 induces tumor growth, stem cell characteristics, metastasis and chemo resistance, an engineered bivalent NRG-1β (NN) could protect against cardiotoxicity in cancer patients treated with doxorubicin [57]. Studies on circumstances under which NRG-1 functions to enhance regeneration and tissue repair and enhancement of tumor cell growth are urgently needed to elucidate the multidimensional aspects of its functional role in health and disease.
Neuromuscular disorders refer to diseases of the nerves that control muscles and communication between nerves and muscles [58]. Myelin plays an essential role in accurate neuromuscular communication and conduction of electrical impulses by axons in both the central nervous system (CNS) and peripheral nervous system (PNS). PNS myelin development depends on axonal signals which are provided by NRG1/ErbB receptors [59]. Similarly, NRG-1 plays a significant role in Hirchsprung disease, in which mutations in NRG-1 gene can lead to absence of ganglion cells along the intestines, leading to obstruction of bowel function [60]. These ganglion cells are part of the enteric nervous system (ENS), for which studies have shown that NRG-1 also plays an important role in [61,62].
During embryonic development, the formation of muscular tissue is carried out through a process known as myogenesis. Recently, it was shown that NRG-1β plays a protective role to improve impaired myogenesis through PPARγ activation and transcriptional activity of NF-κB [63]. This was the first insight into the function of NRG-1β in regulating PPARγ/NF-κB signaling during myogenesis using an in vitro whole serum-based sepsis model.
Understanding of this process is essential for designing future approaches for the treatment of skeletal muscle diseases and the prevention of or recovery from muscle loss in situations such as cachexia, muscle wasting, sarcopenia and other neuromuscular degenerative diseases.
Stroke, transient ischemic attack and intracerebral hemorrhage are cerebrovascular diseases which affect blood supply to the brain [15]. The lack of blood flow during a stroke leads to a complex pathophysiological response, resulting in neural injury. The mechanisms that lead to neural cell loss and injury include cytotoxicity, free radical release and inflammatory changes [18]. The inflammatory response enhances the ischemic injury, but also promotes recovery. Following a stroke, immune cells release proinflammatory cytokines and free radicals which increase the inflammatory response and contribute to the neuronal injury post ischemia [18]. Treatment failure may be due to the intricate pathophysiological response to ischemic injury. Targeting multiple molecules in this pathway remains a treatment challenge.
During ischemic brain injury, NRG-1 provides neuroprotection. This effect, demonstrated by in vitro and in vivo models, is achieved through NRG-1's stimulation of the PI3-kinase/Akt pathway. Oxygen glucose deprivation (OGD) is an in vitro method used to stimulate neuronal cell injury and imitates the pattern that occurs following ischemic injury in vivo [64]. Rat B35 neurons subjected to (OGD)/reoxygenation were treated with NRG-1 and showed significant increases in neuronal survival [65]. Different concentrations of NRG-1 added to neuronal culture at reoxygenation induced protection against OGD-induced neuronal apoptosis [66]. These data show that NRG-1 reduced cell death following OGD in a dose-dependent manner, by activating ErbB4 receptors and GABAergic transmission [23]. Injury to oligodendrocyte progenitor cells (OPCs) caused by hypoxia plays a crucial role in white matter injury. A study observed NRG-1's potential to influence the survival of OPCs damaged by OGD. OPCs were exposed to OGD, then treated with NRG-1 and the results showed that NRG-1 inhibited OGD-induced apoptosis and increased the survival rate of OPCs [67].
In vivo, NRG-1's neuroprotective capabilities were tested via permanent middle cerebral artery occlusion (pMCAO) in an ischemic rat model. NRG-1 treatment prior to pMCAO significantly decreased infarction volume. MK-801, a N-methyl-D-aspartate (NMDA) receptor antagonist, shows similar outcomes to NRG -1 treatment, reducing neuronal death [67]. Co-administration of NRG-1 and MK-801 showed synergistic effects on the reduction of infarct following pMCAO. These findings indicate that NRG-1 has the capability of inhibiting neuronal damage in rat pMCAO models and that combination treatment of NRG-1 with MK-801 enhance the neuroprotective effects. In transient middle cerebral artery occlusion (tMCAO) model, NRG-1 given prior or 13.5 hours after the onset of ischemia in rats reduced cortical damage and improved neurological outcomes [68]. Current treatment of stroke relies on TPA, which is focused on reducing infarct and inflammation associated with the infarct.
Traumatic brain injury (TBI) physical symptoms include loss of consciousness, headache, fatigue and dizziness. The cognitive symptoms include mood swings, memory or concentration problems, and feelings of depression or anxiousness. Earlier studies showed that administration of NRG-1 prior to brain trauma in a mouse model improved retention of spatial memory, suggesting that NRG-1 may improve some functional outcomes after brain injury [69]. Often, BBB disruption occurs following TBI, leading to brain edema, inflammation, and neuronal death. BBB compromised integrity exposes the brain tissue to substances that are present in blood vessels and it disrupts the ionic gradients necessary for proper neuronal function, which may lead to acute and long-term CNS dysfunction [70]. Studies suggest that NRG-1 has beneficial effects on endothelial permeability and BBB permeability following experimental trauma in mice and may have neuroprotective potential during CNS injury [71]. Along with injuring neurons, brain trauma also damages the axons, often resulting in diffuse axonal injury. Axonal injury is one of the most important pathological features following TBI [41]. NRG-1 plays an integral role in axon physiology by influencing the survival and migration of Schwann cell precursors as well as remyelination and functional recovery after injury [42]. In an animal model of spinal cord injury, exogenously administered NRG-1 enhanced the proliferation and differentiation of spinal neural precursor cells into oligodendrocytes, enhanced axonal preservation, and decreased astrogliosis and tissue degeneration [43]. Using a severe sciatic nerve injury model, a study has shown that in NRG-1 mutant mice, axons remyelination was impaired for 2 months after the nerve transection and anastomosis. However, by 3 months post-injury, the axons returned to normal. These data suggest that NRG-1 promotes nerve repair in the early phases of injury [72]. NRG-1 also induced bone pain-like behavior in rats by induction of and activation of ErbB2, Akt-1 and p38MAPK, which could be blocked by ErbB2 inhibitor [73]. The mechanisms involved in nerve repair in the late phases of injury remain to be investigated.
Neuropsychiatric Disorders (ND) affects around 450 million people worldwide. Those inflicted by these diseases tend to have memory, pattern separation, and reward processing problems [74-76]. ND impairs executive functioning, emotional regulation, and social facilitation. These neurobehavioral functions occur because of circuits that reside in the prefrontal cortex [77]. By 2020, it is predicted that ND will be the second highest cause of global disease burden [78].
Studies are recently investigating the pivotal role of NRG-1 and inflammation in the development of the cognitive disorders. NRG-1 plays an important role in brain plasticity and development. Moreover, NRG-1 has been found in multiple regions of the adult brain and is involved in the regulation of neurotransmission [79]. ND occurs in individuals with neurodevelopment problems and limited plasticity. In vivo studies using mouse models have focused on increasing and decreasing the NRG-1 and ErbB network levels. Results showed many behavioral deficits that suggested modified signaling intensity, which corresponds to a pathophysiological mechanism of psychosis and ND [80]. Inflammation can be defined as a part of the immune response that occurs after a type of physical injury or microbial invasion [81]. Research suggests that inflammation may play an important role in cognitive disorders and needs to be studied in greater detail in ND patients [82].
The main ND that present a correlation with inflammation and NRG-1 are Schizophrenia (SZ), Bipolar Disorder (BD), and Alzheimer's Disease (AD). Evidence for increased inflammation has been found in a study that included 3,952 adolescents diagnosed with BD, Autism Spectrum Disorder (ASD), and SZ. Proinflammatory markers (PIMs) were measured and showed to be elevated in those diagnosed with ND. However, this study suggested that future research is needed, using a larger population with one specific diagnosis [76].
SZ is an extremely debilitating disorder that has afflicts about 1% of the population [11]. Symptoms of this disorder include a misinterpretation of reality, hallucinations, disordered thinking, and an overall impairment of daily functioning that results from behavior changes. NRG-1 has been one of the most extensively studied genes in SZ [80]. One study conducted in 2017 examined the levels of NRG-1 and EGF in treatment-resistant schizophrenic patients and found decreased or abnormally expressed levels of these proteins [79]. While past research has examined the levels of NRG-1 in the brain [83], future research could look toward understanding the mechanisms regulating NRG-1 expression and function and possible use of NRG-1 as a treatment for ND disorders. NRG-1 has also been used to suppress the induction and the expression of long-term potentiation in the hippocampus. In another mutant mice study, looking at the influence of NRG-1 and ErbB4, researchers observed a trend of loss of function in specific genes that specifically show to be risk factors for SZ [11]. This finding raises the question of a possible disease-related pathophysiological significance, perhaps inflammation. A study conducted on a group of post-mortem human schizophrenic brains have shown differential expression of NRG-1 mRNA and protein in many different regions of the brain, most notably seen in dorsolateral prefrontal cortex and the hippocampus [79]. This differential expression of these pathways suggests that NRG-1 is an interesting target to examine for SZ research.
In BD, individuals have an impairment of multiple cognitive abilities and functions, which corroborates a potential role of neuroinflammation [84]. Macrophages, or microglia, located in the brain, become activated in response to any kind of damage, leading to inflammation [81]. Inflammation is responsible for influencing the shaping process of synaptogenesis (the formation of new synapses) and the overall reduction of neurons [81]. The link between dysfunctions, cognitive impairment, medical comorbidity and premature mortality seen in BD patients can be explained by immune system signaling the microglia [81]. In both BD and SZ studies, NRG-1 deficiency has resulted in behavioral deficits such as working memory impairments in both disorders [74]. There has been much overlap in the diagnosis of BD and SZ because of the similarity they have. Research has found that over 50% of those diagnosed with BD have some other kind of comorbidity [81-84], which makes the differentiation between BD and SZ difficult for clinicians. The biggest difference found between the two ND is in regard to the neuropsychological deficits and the time at which they present themselves: SZ individuals exhibit dysfunction preceding the onset of the illness, which becomes greater during the first few years of being diagnosed, while BD patients show cognitive development pre-morbidly and demonstrate first episodes that are extremely intense [84]. These key differences of the timing of the presentation of symptoms have been likened role of inflammation in BD and SZ [85], along with abnormal sequences and patterns of NRG-1 and other proteins. A study suggested that NRG-1 deficiency regulates microglial cells that are stress induced [74]. Thus, NRG-1 could be used as treatment because of its anti-inflammatory properties.
Studies on Alzheimer's disease (AD) have shown that neuroinflammation plays a role in this disease [12,86,87], thus NRG-1 is considered a candidate for its treatment [6]. NRG-1 is responsible for creating extremely complex networks of signaling proteins with many differing expressions and divergent functions during the development of an adult nervous system [86]. During inflammation, these proteins alter their function. Incorrect or low expression of these proteins lead to manifestation of AD symptoms [74]. NRG-1 deficiencies have been shown to provide evidence for the neuropathology of psychosis [85]. In AD brains, ErbB4 immunoreactivity was shown to colocalize with apoptotic Bax signal in apoptotic hippocampal neurons. Thus, the upregulation of immunoreactivity of NRG-1 receptor ErbB4, may be involved in AD progression [32]. Another study showed that NRG-1 upregulated the expression of anti-apoptotic protein Bcl-2, demonstrating the neuroprotective potential of NRG-1 in AD [88]. The soluble NRG-1 plasma levels were significantly higher in mild and moderate AD patients compared to control patients, indicating that NRG-1 could be used as a marker for early diagnosis of AD [89]. Due to its localization in the brain areas relating to auditory and visual hallucinations and delusions, it has been hypothesized that the presence of NRG-1 may interact with specific inflammatory pathological processes that have been shown to be associated with Late Onset Alzheimer's Dementia (LOAD) [85]. Furthermore, a study suggests that NRG1 plays a role in increasing the genetic risk to positive symptoms of psychosis in a proportion of LOAD families [85]. However, these results are controversial, since a different study [90] found no correlation between NRG-1 variants and delusions, hallucinations, psychosis, or elation/mania in an AD cohort. Further investigations are required, and large longitudinal patient cohorts should be used.
Malaria, caused by Plasmodium (with P. falciparum being the deadliest), is a devastating infectious disease causing approximately 207 million cases worldwide and 627,000 deaths a year, mainly in children under 5 years of age living in sub-Saharan Africa, [91]. In the trophozoite-stage, intra erythrocyte (RBC) parasite proteins are transferred to the surface of the RBC where they play a role in adherence to the host's endothelium [92,93], destruction of RBC's and release of cytotoxic heme moieties into circulation which together with interactions with host cells [94,95] mediate severity of the disease.
Approximately 1% of P. falciparum infections results in cerebral malaria (CM), a brain encephalopathy associated with malaria especially in children [91]. In adults in South-East Asia, CM accounts for 50% of the malaria deaths, as they not only suffer from encephalitis, but also have multiple organ failure [96]. CM is clinically defined as having P. falciparum parasitemia and unarousable coma, ruling out any other causes, such as meningitis.
Recently there has been an awareness of the burden of varying neurological deficit in survivors [97]. CM-related neurological sequelae are more severe in children than in adults. Children living in Africa with severe neurological sequelae often die within a few months of discharge [98]. In adults, coma depth and duration along with multiple convulsions are independent risk factors for neurological sequelae [98]. These include cranial nerve lesions, neuropathies, and extrapyramidal disorders [99].
The pathogenic mechanisms leading to fatal human CM are multifactorial and involve alterations in cytokine and chemokine expression, localized inflammation and microvascular injury that results in the loss of blood brain barrier (BBB) integrity. Two hypotheses have been proposed to explain dysfunction of the BBB and mortality associated with CM [100]. First, the sequestration hypothesis, proposes that infected red blood cells (iRBCs) bind to endothelial cells and accumulate within brain microvessels [101-103] obstructing blood flow, resulting in low tissue perfusion, compromised oxygenation and brain tissue damage. Second, the immunopathological "cytokine" hypothesis, suggests that an exaggerated host immune response to Plasmodium infection and the adherence of parasitized erythrocytes to endothelial cells promotes the release of pro-inflammatory mediators and other cytotoxic molecules responsible for compromising BBB cellular components, resulting in edema, access of toxins to sensitive brain tissue, neuronal cell damage, coma and death [104-106]. However, pathogenesis of CM has not been shown to be exclusive to any one hypothesis but involves a combination of pathophysiological features, including parasite sequestration in the brain, metabolic disturbances and host inflammatory responses that mediate CM pathogenesis [104-106].
There are pathophysiological similarities between CM, acute ischemia syndrome (AIS) and TBI, including an exaggerated host expression of pro-inflammatory factors. This inflammatory response leads to increased microvascular endothelial activation with upregulation of adhesion molecules, glial activation, focal inflammation and activation of apoptotic pathways, which can eventually lead to brain damage, coma, and death. Recognizing fundamental similarities shared by CM pathogenesis and both AIS and TBI, an experimental cerebral malaria (ECM) model (Plasmodium berghei ANKA in C57BL/6 mice) was used for the first time to investigate the role of NRG-1 in ECM prognosis. The results indicated that ECM was associated with depletion in circulating levels of (NRG-1β) [107]. Indeed, intravenous infusion of NRG-1 at onset of ECM attenuated ECM mortality by stimulating a robust anti-inflammatory response, while reducing pro-inflammatory factors. Also noted was the reduction of accumulation of infected erythrocytes in brain microvessels, reduction of brain tissue damage, and reduction of total mortality.
We recently showed that NRG-1 protects human brain microvascular endothelial cells (hCMEC/D3) and astrocytes from cell death induced by CXCL10 and heme (a cytotoxic by product released by damaged infected and non-infected erythrocytes) in vitro [21,22] In addition, NRG-1 improved heme-disrupted BBB integrity in an in vitro BBB model consisting of hCMEC/D3 and human astrocytes. In the, cortex and hippocampus of mice with advanced stages of ECM, the ErbB4 protein is inactivated through dephosphorylation, indicating a diffuse distribution in regions of tissue injury. However, exogenous infusion of NRG-1 increased phosphorylated ErbB4 levels in the cortex and hippocampus of infected mice; this subsequently reduced STAT3 activation (a typical pathogenic pathway in CM) and increased AKT activation. These findings were consistent with Lok's report [108] which showed that NRG-1 increases pAKT in order to enhance in vitro survival of endothelial cells. Overall, it seems that NRG-1 protects against apoptosis and cell death of two major BBB components, human brain microvascular endothelial cells and astrocytes (Figure 2). Thus, NRG-1 attenuates mortality associated with CM through activation of ErbB4/AKT and inactivation of STAT3 signaling pathways. These findings suggest that adjunctively augmenting NRG-1 during ECM therapy may be an effective therapeutic approach to reduce CM-induced CNS tissue injury. To date no other studies with other infectious diseases such as Zika, toxoplasmosis, HIV/AIDS and others with reported deleterious effects on the CNS has been reported. It will be interesting to determine whether NRG will attenuate the CNS injury associated with these diseases and their respective neuropathologies or sequelae.
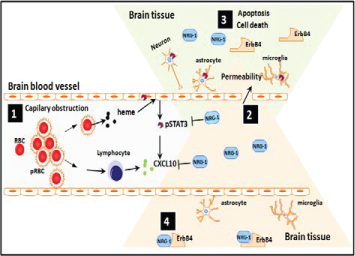 Figure 2: Proposed model demonstrating protective role of NRG1 against cerebral malaria (CM) pathogenesis. During CM pathogenesis, some P. falciparum-infected erythrocytes (pRBC) lyse and release heme while others bind to brain vascular endothelial cells via adhesion molecules (ICAM, VCAM, etc) causing capillary obstruction and activation of endothelial cells as well as release of pro-inflammatory and angiostatic factors such as CXCL10 and activation of STAT3 (1). Downstream of the capillary obstruction, endothelial cells, astrocytes, microglia and neurons located in the penumbra are at risk of undergoing apoptosis which results in increased permeability of BBB (2) due to dead and dying cells in irreversibly damaged brain tissue regions (3). After administration of adequate amounts of NRG-1, cells in the penumbra can be rescued from apoptosis. It is proposed that this might be the basis for effective attenuation of mortality and associated neurological sequelae in patients with CM (4).
View Figure 2
Figure 2: Proposed model demonstrating protective role of NRG1 against cerebral malaria (CM) pathogenesis. During CM pathogenesis, some P. falciparum-infected erythrocytes (pRBC) lyse and release heme while others bind to brain vascular endothelial cells via adhesion molecules (ICAM, VCAM, etc) causing capillary obstruction and activation of endothelial cells as well as release of pro-inflammatory and angiostatic factors such as CXCL10 and activation of STAT3 (1). Downstream of the capillary obstruction, endothelial cells, astrocytes, microglia and neurons located in the penumbra are at risk of undergoing apoptosis which results in increased permeability of BBB (2) due to dead and dying cells in irreversibly damaged brain tissue regions (3). After administration of adequate amounts of NRG-1, cells in the penumbra can be rescued from apoptosis. It is proposed that this might be the basis for effective attenuation of mortality and associated neurological sequelae in patients with CM (4).
View Figure 2
Animal studies have shown that myocardial NRG-1 is activated in response to ischemia. Furthermore, when NRG-1β is used as treatment in animal models of cardiac failure, studies have shown that it improves heart function [109]. In an in vitro study, NRG-1β treatment within the period of deoxygenation and glucose deprivation, significantly increased oligodendrocyte type2 astrocytes progenitor's survival and decreased apoptosis [110]. There is also concern that NRG treatment might exert "off-target" effects by nature of its mitogenic properties as a growth factor, and the well-established role of ErbB2 as an oncogene in a variety of malignancies. To address this issue, a bivalent version of NRG was engineered to preferentially target ErbB4 homodimer activation, thereby preventing doxorubicin-induced cardiomyocyte death without activating or potentiating cancer cell signaling [76,78]. Further studies are clearly warranted to determine whether this or other modified ligands, or alternative delivery methods are needed to increase the therapeutic window for NRG [111]. When NRG-1 was administered for 10 days in patients with heart failure, it improved cardiac function when patients were evaluated after 30 days (ChiCTR-TRC-00000414) [44]. When NRG-1 was infused for 11 days, cardiac function was improved for 12 weeks (Australian New Zealand Clinical Trials Registry, anzctr.org.au Identifier: ACTRN12607000330448) [112]. Currently, clinical trials that use NRG-1 in heart failure are: NCT01214096, NCT01439789, NCT01439893, NCT01251406, NCT03388593, but no results have been yet posted.
NRG-1 has neuroprotective effects against ischemic stroke. Studies suggest that there is a therapeutic window of opportunity between the onset of ischemia and irreversible neuronal death and that irreversible injury is not complete until about 6 hours after ischemia [113]. However, if a single bolus injection of NRG-1 was administered at either 0, 4 or 12 hours after reperfusion following middle cerebral artery occlusion [113], a significant decrease in the subcortical and cortical lesions in NRG-1 treated rats was shown. These results contribute to NRG-1's potential use in therapy in focal cerebral ischemia. When NRG-1β was administered, the ischemia-induced apoptosis decreased, the abnormal morphological structures of nerve cells were ameliorated, the integrity of the BBB was restored, and infarct volume was reduced. At the same time, neurological function was significantly recovered [114]. Cimaglermin (NRG-1β) treatment significantly enhanced recovery after stroke, inducing axonal sprouting and synapse formation. These data suggested that Cimaglermin represents a potential candidate for stroke treatment [115].
Because of its critical contribution, BBB integrity is a key potential therapeutic target in the treatment of the acute phase of brain trauma. A study was carried out in which mice were subjected to controlled cortical impact (CCI) under anesthesia and BBB permeability was assessed by measuring Evans blue dye extravasation [86]. NRG-1 was administered intravenously for 10 minutes following trauma and Evans blue dye extravasation decreased by 35% 2 hours post trauma. The results showed that NRG-1 decreases BBB injury in a model of TBI. This data provides evidence that NRG-1 has a beneficial effect on the barrier function of the brain's microvasculature following trauma. These various studies demonstrate NRG-1's potential role in the therapeutic strategies for traumatic brain injury. Currently, NRG-1 is undergoing clinical trials for use against TBI (ClinicalTrails.gov NCT01258387 and NCT01944683).
In a rat model of Charcot-Marie-Tooth neuropathy, early NRG-1 treatment promoted Schwann cell differentiation, preserved axons and restored nerve function [116]. In a mouse model of spared nerve injury, NRG-1 inhibited neuropathic pain in a dose-dependent manner. However, in a formalin-induced pain model, NRG-1 was found to aggravate pain. These findings may provide different approaches for neuropathic pain in different injury types [117]. Conversely, monoclonal antibody Herceptin (Trastuzumab) blocked NRG receptor ErbB2 and increased axonal regeneration in a rat model of acute or chronic nerve injury. However, these effects were independent from NRG pathway. These results raise the possibility of using targeted therapies to improve outcomes of peripheral nerve injury [118].
NRG-1 could potentially significantly increase the effectiveness of current anti-malarial therapy against human CM. Despite appropriate anti-malarial treatment, mortality associated with CM remain as high as 30% while up to 20% of survivors experience neurological complications [96]. Thus, anti-malarial drugs are clearly not adequate for saving lives of CM non-survivors and adjunctive therapy is needed. Adjunctive therapy has been proposed based on pathophysiology studies conducted with murine ECM. The purpose of these adjunctive therapies were to shorten coma and decrease fatality as well reduction in the rate and severity of neurological sequelae [98]. Some of these therapies have been deleterious but others are currently being explored, NRG-1 being one of them.
Evidence from genetic, transgenic and post-mortem studies have strongly suggested that altered NRG-1/ErbB4 signaling is involved in SZ susceptibility. NRG-1/ErbB4 signaling has interactions with GABAergic, glutamatergic and dopaminergic neurotransmissions that are involved in SZ. Identifying the targets in NRG-1/ErbB4 signaling and interactive pathways will provide opportunity for development of antipsychotics with specific efficacy and fewer side effects [119].
Neuregulin can be used as treatment in other diseases. Neuregulin regulates glucose and lipid homeostasis. An engineered fusion protein using NRG-1 and Fc domain of human IgG1 (NRG1-Fc) exhibits extended half-life in circulation and improved potency in receptor signaling. NRG1-Fc treatment lowered blood glucose, improved insulin sensitivity and suppressed food intake in obese mice [120]. In a different study, NRG-1 treatments improved glucose tolerance in Db/Db mice, through inhibition of hepatic gluconeogenesis [121].
Studies have shown that in lung cancer animal models, inhibition of NRG-1 signaling leads to reduced tumor growth and enhanced intensity and duration of the response to chemotherapy [122]. Furthermore, clinical trials especially used drugs that targeted the ErbB (HER) receptors. In a Phase IV clinical trial that enrolled patients with advanced HER+ carcinomas, Lumretuzumab (anti-HER3 monoclonal antibody) was used in combination with EGFR-blocking agent Erlotinib or Cetuximab. The toxicity of those drugs was manageable, but there was no evidence of meaningful clinical benefit [123]. However, Duligotuzumab, a dual-action antibody that blocks ligand binding to human HER3 and EGFR, in combination with chemotherapy (Cisplatin-5-Fluorouracil or Carboplatin-Paclitaxel) was used for patients with recurrent or metastatic squamous cell cancer of the head and neck. This treatment combination showed encouraging activity, 67% of the patients confirming partial or complete response [124].
Clinical trials targeting NRG pathways in cancer use MM-121 (antibody targeting ErbB3) in combination with chemotherapy in Heregulin-positive non-small cell lung cancer (NCT02387216). A Phase 2 trial of MM-141 (antibody against Her3 and IGF-1) and Nab-paclitaxel and Gemcitabine in pancreatic cancer is active, but not recruiting yet (NCT02399137). In ovarian cancer, study #NCT01447706 tried to determine whether the combination of MM-121 and paclitaxel has better results than paclitaxel alone. Results showed that overall survival was 13.7 months for Pax + MM141 compared to Pax alone (10.17 months). In cancer, targeting NRG pathway seem to improve survival and prognosis.
Studies have shown the importance of NRG and its corresponding receptors in organ development and maintenance, as well as in many disorders. However, the functions of NRG-1 and the pathways it mediates are not completely understood; Figure 3 shows proposed NRG-1 pathways. In animal models, homozygous deletion of the NRG-1 gene is devastating, leading to death in utero, overexpression of NRG-1 impacts negatively on various cognitive and behavioral processes, suggesting that further investigations are necessary to determine the NRG-1 dose that is ultimately beneficial for patients.
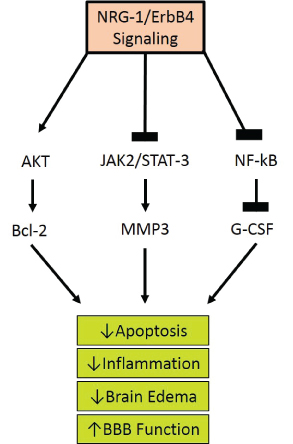 Figure 3: Proposed Neuregulin-1 Pathways. The ligand interaction with ErbB 1,3, and 4 increases their affinity and induces heterodimerization of ErbB1-4, activating the tyrosine kinase domain. This allows the cytoplasmic region of the ErbB to become phosphorylated. The phosphorylated tyrosine residues recruit various adaptors/effectors that induce intracellular signals leading to the survival of the cell by lowering apoptosis, inflammation, edema, and ultimately restoring BBB function.
View Figure 3
Figure 3: Proposed Neuregulin-1 Pathways. The ligand interaction with ErbB 1,3, and 4 increases their affinity and induces heterodimerization of ErbB1-4, activating the tyrosine kinase domain. This allows the cytoplasmic region of the ErbB to become phosphorylated. The phosphorylated tyrosine residues recruit various adaptors/effectors that induce intracellular signals leading to the survival of the cell by lowering apoptosis, inflammation, edema, and ultimately restoring BBB function.
View Figure 3
Although NRG-1 plays a protective role during development and adult life, its effects in disease vary. NRG-1 improves cardiac function in patients with chronic heart failure and increases tissue viability and angiogenesis in animal models of heart ischemia. Furthermore, NRG-1 is neuroprotective during episodes of brain ischemia and injury, as well as in AD. Its elevated plasma levels in patients with mild or moderate AD suggests that NRG-1 can be used as a marker for early diagnosis. However, the role of NRG-1 in cognitive disorders remains to be fully understood. In ECM, we demonstrated that NRG-1 circulating levels diminished, while its infusion led to a reduced mortality due to a strong anti-inflammatory response and a reduction of parasitic RBC accumulation in microvessels and decreased tissue damage. Furthermore, we showed that NRG-1 protects brain microvascular cells, astrocytes and BBB integrity from heme, a product released by damaged erythrocytes. In addition, we demonstrated that NRG-1 effects on ECM were induced through ErbB4/AKT and STAT3 pathways. Although NRG-1 has protective roles in noninfectious cardiovascular and neurological disorders, as well as in infectious diseases, such as malaria, its role in cancer is completely different. It has been demonstrated that in cancer, NRG-1 induces tumor growth, stem cell self-renewal and chemoresistance.
Due to its multiple functions in health and disease, NRG-1 could be used either as treatment or as a target in clinical trials. Future studies will open new opportunities of research and potentially increase the success of using NRG-1 in treatment regimens.
JKS is funded by the National Institute of Neurological Disorders and Stroke NIH/NINDS R01 NS091616 and R21 TW006804 as well as 8G12MD007602 from the National Institute of Minority Health and Health Disparities (NIMHD). The funding bodies had no involvement in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMHD or the NIH.