The glucagon-like peptide-1 (GLP-1) is an endogenous hormone produced by the L cells in the intestine. It is released in response to the intake of food and intestinal dilatation. It stimulates the pancreatic beta-cell to secrete insulin which is dependent upon ingestion of food. GLP-1 acts to slow down gastric motility and suppress appetite through the GLP-1 receptors in the satiety center [1]. It is neutralized by the dipeptidyl peptidase-4 (DPP-4) enzyme very quickly. Hence the endogenous GLP-1 has a shorter duration of action.
The pharmacological preparations of GLP-1 receptor agonists (GLP-1RA) are immune to the activity of the DPP-4 enzyme, thus achieving a significantly higher plasma levels than the endogenous GLP-1. GLP-1RA which are non-insulin injectables have gained a unique place in a select cohort of patients with high BMI (Body Mass Index) due to the therapeutic benefit which includes weight loss and reduction in HbA1c [2]. The most commonly used are once-a-week injectable preparation due to the obvious benefits of reduced injection burden in comparison to once-daily preparations. The recent cardiovascular outcome trials involving Liraglutide, semaglutide, and dulaglutide concluded the reduced cardiovascular risk in comparison to placebo [3-5]. The availability of an oral formulation of GLP-1RA expands the therapeutic option.
Oral semaglutide is the first oral GLP-1 RA. The new formulation has been approved by the European medicines agency and the United States Food and Drug Administration (FDA) for use in the management of Type 2 diabetes.
It can be used for the treatment of adults with insufficiently controlled type 2 diabetes mellitus to improve glycaemic control as an adjunct to diet, exercise and
1. As monotherapy when metformin is considered inappropriate due to intolerance or contraindications.
2. In combination with other medicinal products for the treatment of diabetes.
Oral Semaglutide is a tablet formulation for once-daily administration. It is co-formulated with an absorption enhancer, sodium N- (8-[2-hydroxybenzoyl] amino) caprylate (SNAC). SNAC is a small fatty acid derivative that promotes the absorption of semaglutide across the gastric mucosa. It induces a transient, localized increase in gastric pH and protecting the semaglutide molecule from the proteolytic effect of very low gastric pH. The net result is a greater concentration of semaglutide at the mucosal surface leading to transcellular absorption across the gastric epithelial surface into circulation. It was noted that following oral administration, maximum plasma levels are reached after 1 hour. Steady-state plasma concentrations are achieved after 4-5 weeks of use [6]. This mechanism is shown in Figure 1.
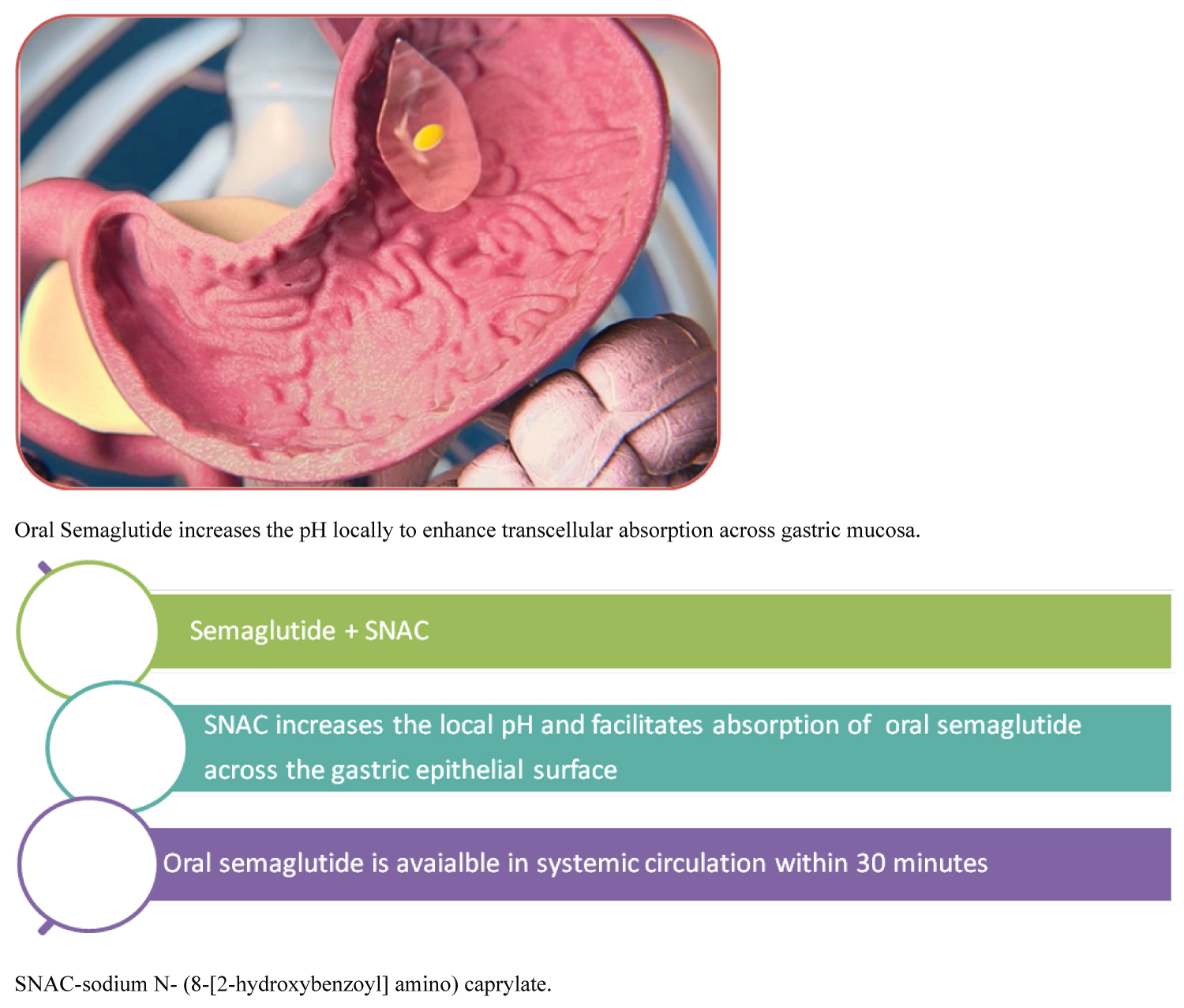 Figure 1: Mechanism of action of semaglutide (reproduced with Permission from Novo Nordisk Pharmaceuticals). View Figure 1
Figure 1: Mechanism of action of semaglutide (reproduced with Permission from Novo Nordisk Pharmaceuticals). View Figure 1
Pioneer group of trials compare and analyze the effects of oral semaglutide as an adjunct to currently available treatment options in managing type 2 diabetes. The primary endpoints of the trials were the Hba1c reduction and weight loss. It has shown benefits of higher Hba1c reduction and weight loss when compared with Placebo, Sodium-Glucose Transporter-2 inhibitors (SGLT2i), and DPP-4 inhibitors [7-10]. The oral semaglutide at 7 mg and 14 mg doses showed the highest reductions in Hba1c reduction and weight loss from baseline. Pioneer 4 trial compares the effect of oral semaglutide with liraglutide subcutaneously. At the end of 26 weeks, the Hba1c reduction was similar to liraglutide but the weight loss achieved was significantly higher with oral semaglutide [10].
Pioneer 8 trial showed the beneficial effects of adding oral semaglutide to patients treated with ongoing basal insulin therapy. There were significant Hba1c reduction and weight loss along with the reduction in the total dose of insulin required when compared to the placebo arm. The basal insulin was reduced by 20% at the start of the trial and the oral semaglutide arm showed further reduction in the total dose of insulin required. The 7 mg and 14 mg doses were associated with maximum beneficial outcomes [11].
According to FDA, new therapeutics for type 2 diabetes have to show cardiovascular safety data. The pioneer 6 trial is the cardiovascular outcome trial, which is an event-driven double-blind, placebo-controlled, randomized study in patients with high cardiovascular risk. The composite MACE (nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death) was numerically lower in the semaglutide group. The study confirms the non-inferiority of semaglutide when compared to placebo [12].
A known complication of type 2 diabetes is nephropathy characterized by albuminuria and progressive reduction in glomerular filtration rate. Pioneer 5 trial analyzed the efficacy of oral semaglutide in patients with moderate renal impairment classified based on eGFR 30-59 ml/min per 1.72 m2. Patients taking 14 mg of oral semaglutide had a significant reduction in HbA1c and achieved significant weight loss compared to the placebo group. During the 26 weeks trial period the renal function remained unchanged in both groups [13]. Oral semaglutide treatment was associated with more gastrointestinal side effects as expected with GLP-1 RA therapies. Granhall, et al. conducted a pharmacokinetic study using oral semaglutide in patients with renal function ranging from normal function, mild to moderate renal impairment, severe and end-stage renal impairment on hemodialysis. The area under the curve (AUC) and plasma maximum concentration Cmax at day 10 did not vary across the renal function groups. The renal impairment does not affect the pharmacokinetics of oral semaglutide [14]. It is not recommended for patients with end stage renal disease.
Oral semaglutide is available in 3 mg, 7 mg, and 14 mg tablets. The initiation dose is 3 mg per day - 1 month and increase the dose to 7 mg/day. The dose can be further titrated up to a maximum of 14 mg per day if required to achieve glycaemic control [15].
Initiate 3 mg of Oral semaglutide
⇩
After 4 weeks: Increase the dose to 14 mg once a day
⇩
After 4 weeks: Increase the dose to maximum 14 mg once a day if tolerating well.
It should be consumed on empty stomach along with a maximum of 120 mL (4 ounces) of water. It is essential to avoid any food, beverage, and other medications for at least 30 minutes following the oral semaglutide. If a dose is missed, then the next dose should be taken the next day. To ensure the efficacy of the tablet, it should not be crushed or chewed and is taken as a whole.
One of the key questions is who should be offered oral semaglutide? The current guidance from ADA/EASD (American Diabetes Association/European Association for the Study of Diabetes) recommends considering existing risk factors like Heart failure, Chronic kidney disease, Body Mass Index, and hypoglycaemia risk before choosing the next therapeutic agent after initial treatment with metformin. Usually, patients with higher BMI, suboptimal glycaemic control, and with existing cardiovascular disease or with high-risk factors will benefit from GLP-1 analogs and SGLT2 inhibitors [16].
Type 2 diabetes patients with preference to oral agents and those with needle phobia would benefit the most from oral semaglutide. Patients who cannot self-inject or those with have poor injection technique should be offered oral semaglutide where appropriate. The National Institute for healthcare and Clinical Excellence (NICE) Guidance criteria for GLP-1 prescription should be followed and it is prudent to apply the same criteria for oral semaglutide [17].
The oral semaglutide is novel and can be preferred for its ease of use. However, the absorption enhancer (SNAC) and semaglutide have the potential to interact with other concomitant oral medications.
The current practice is to use levothyroxine on empty stomach away from food, medications, or strong coffee for at least 30 to 45 minutes. The pharmacokinetic studies were conducted to analyse the interaction following administration of a single dose of levothyroxine (600 mcgm) with oral semaglutide 14 mgs at a steady state. The study showed an impact on the pharmacokinetic profile of thyroxine demonstrating a 33% increase in thyroxine area under the curve [18]. The increase can be attributed to the delayed gastric emptying effect of semaglutide and consequent increased gastric absorption of levothyroxine. In patients who are treated concomitantly with levothyroxine, the interaction with oral semaglutide should be discussed. Levothyroxine has a 24-hour profile and the early morning dosing versus bed-time dosing should not cause any significant impact on its pharmacokinetic profile and clinical outcomes. The trials conducted have shown improved thyroxine levels with bedtime Levothyroxine dosing [19,20].
It would be a pragmatic approach to change the timing of levothyroxine dosing to bedtime, at least 2-3 hours after the evening meal. The clinicians can consider this option whilst co-prescribing oral semaglutide along with levothyroxine.
Proton pump inhibitors (PPI) are recommended to be used on empty stomach to increase the gastric pH. The regular use of PPI increases the gastric pH in both fasting and fed states. The study by Baekdal, et al. analysed the impact of omeprazole on the absorption of semaglutide. Omeprazole with oral semaglutide administration was associated with a slight, but not statistically significant, increase in plasma levels of semaglutide when compared to oral semaglutide taken alone [21]. Therefore they can be co-administered without any dose adjustments to PPI's.
In the study conducted by Baekdal, et al. among healthy individuals, Oral semaglutide did not alter the pharmacokinetics of the commonly prescribed medications like lisinopril, metformin, digoxin, and warfarin. There was an increased time under the area of curve (AUC) for metformin however the maximum plasma concentration (Cmax) was not significantly affected. Hence it is safe to use these commonly used concomitant medications along with oral semaglutide [22].
The main adverse effect of oral semaglutide is gastrointestinal upset commonly presenting with nausea, vomiting, and abdominal discomfort [23]. This is similar to observed side effects with injectable GLP-1 RA. They should be used with caution in patients with a history of pancreatitis due to the rare case reports of such occurrence [24,25].
The risk of worsening in diabetic retinopathy has been reported in clinical trials with GLP-1 analogs. Oral Semaglutide should be used with caution in insulin-treated patients who have established diabetic retinopathy. However, a recent experimental and clinical study showed no increase in retinal angiogenesis with GLP-1 analog exposure [26]. The safety data for use during pregnancy and lactation is not available. Oral semaglutide should be stopped 2 months before a planned pregnancy. Women of childbearing age should use contraception whilst taking oral semaglutide [27].
In the rapidly changing landscape of type 2 diabetes management, oral semaglutide is the first oral preparation of GLP-1RA. It can be used as an add-on to ongoing anti-diabetes treatment to achieve individualized targets. It would be prudent to reach the full dose of 14 mg once a day if tolerated well and when additional glycaemic control is preferred. The clinicians should take into consideration the unique formulation and its possible effect on the absorption of other oral medications.
No funding has been received for writing this article.
Dr. V Eligar has received speakers' honorarium from Astra Zeneca, NAPP pharmaceuticals, Boehringer Ingelheim and Eli Lilly. He has received conference grants from Novo Nordisk.
• First available oral GLP-1 analog
• Unique preparation with absorption enhancer for once-daily administration
• To be taken on an empty stomach with water
• Allow at least 30 minutes before other medications, food, or beverage for the best response
• Can be combined with other ongoing anti-diabetes drugs except for DPP-4 inhibitors
• Caution in patients with a history of pancreatitis
• Manage concomitant prescribed medications with a pragmatic approach. Example: Instruct to take Levothyroxine at night if possible.