Aim: To study the prevalence of Cyclin D1 expression and its correlation with other clinicopathological parameters like age, tumor size, lymph node status, MBR grade, and expression of other immunohistochemical markers like ER, PR, Her 2 - neu, and Ki67.
Material and methods: Thirty cases diagnosed were included in the study. All the cases were subjected to routine histopathology along with IHC for ER PR, Her2-neu, Ki67, and Cyclin D1.
Results: CylinD1 positivity was found in 70% of cases. Our study presented the following distribution of cases Luminal A, N = 6(20%), Luminal B, N = 5(16.7%), Triple-negative, N = 10(33.3%), Normal Like = 7(23.3%), Her2/neu enriched = 2(6.7%). N = 4(40%) of triple-negative molecular subtype were positive for the cyclin D1. Cyclin D1 positivity showed a strong correlation with ER, PR, Her2-neu, and Ki67 status. However, no other significant correlation was found with other factors.
Conclusion: This study shows a positive correlation of CyclinD1 with many other prognostic markers like molecular subtype, ER, PR, Her2/neu, and Ki67. With the advent of CD4/6 inhibitors and their potential therapeutic effects in breast carcinoma, Cyclin D1 becomes a potential prognostic IHC marker and can aid in patient management in cases of breast carcinoma. Its association with other established markers and clinicopathological parameters needs to be researched thoroughly.
Breast cancer is the most common cancer in women worldwide [1]. Breast cancer is composed of heterogeneous molecular groups with different prognosis. With increased understanding of the molecular mechanism and their targeted therapy outcomes are expected to improve. Cyclin D1 is the product of the CCND1 gene located in chromosome 11q13 and is an important regulator of the cell cycle [2]. It is a rate-limiting step in the cell cycle progression. It binds with cyclin-dependent cyclin kinase (cdk4/6) and by the inactivation Rb gene, it thus helps in the progression of the cell cycle [3]. Its aberrant expression causes breast carcinogenesis by cell cycle mediated action. In this study, we attempted to correlate its expression with various other clinicopathological prognostic parameters.
A total of 30 cases were included in the study who presented in the tertiary care center in the span of one year after approval from the institutional ethical committee. The clinical data of all the cases were collected from biopsy requisition slip. Patients with incomplete data were excluded from the study. Specimens received were processed as follows. After fixation, grossing and tissue processing 3-5 um thick sections were stained with hematoxylin and eosin (Figure 1). The stained slide was reported according to the Cap protocol for invasive breast carcinoma. Section of the primary tumor showing the highest grades were selected for immunohistochemistry (IHC).
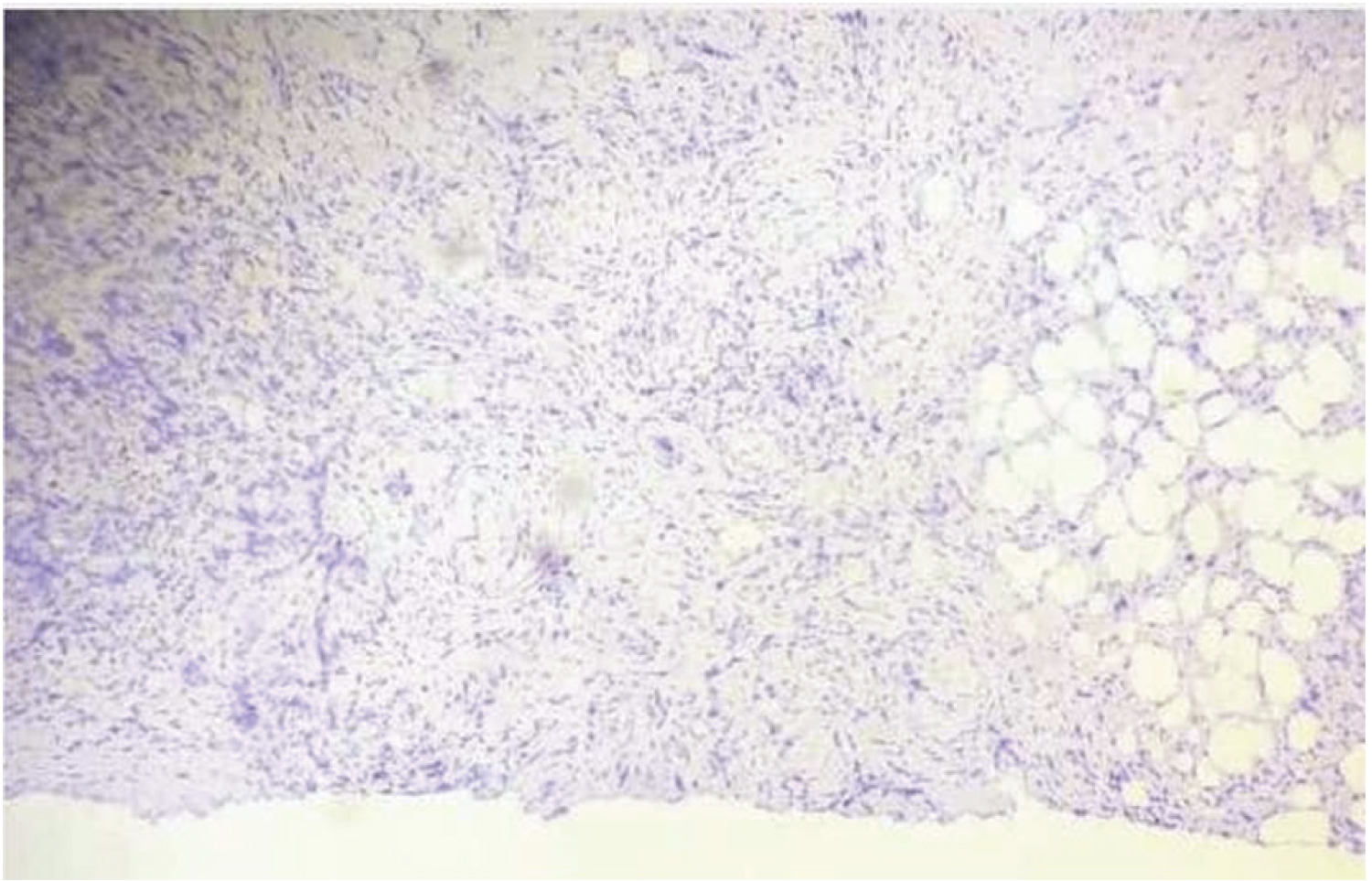 Figure 1: H&E stain of breast tumour 20X.
View Figure 1
Figure 1: H&E stain of breast tumour 20X.
View Figure 1
Primary antibodies used were anti-human cyclin D1 (Clone EP12, Dako), Monoclonal Mouse Anti-human Ki67 Antigen MIB1 along with Monoclonal Rabbit Anti-human Estrogen receptor alpha clone EP1, Ready to use progesterone and Antihuman Her2/neu oncoprotein CB 11 antibody. 3-5 μ thick sections were dewaxed, rehydrated and epitope retrieval was done in a pressure cooker at 15 psi and 120°C for 10 min by incubating slides in citrate buffer (pH 6.0). Hydrogen peroxidase was used for blocking endogenous peroxidase activity. Thereafter primary antibody was added and incubated for 1 hour and subsequently treated with biotinylated secondary antibody for another 30 minutes. This step was followed by treating the slide with Streptavidin horseradish peroxidase for 10. Diaminobenzidine (DAB) was used as the chromogen and counterstaining with hematoxylin was carried out. Staining for cyclin D1 was interpreted as positive when 10% or more tumor cells showed moderate to strong nuclear staining (Figure 2 and Figure 3) [4]. Staining was scored as 0 -3, 0 = negative staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining [5]. ASCO and CAP guidelines were used for ER, PR, Her2/neu and percentage positivity of ki67 was broadly divided in to > 14% and < 14% for assessing results [6]. Data analysis was performed using SPSS 26.0 for Windows student version. Statistical tools like Chi-square test were applied.
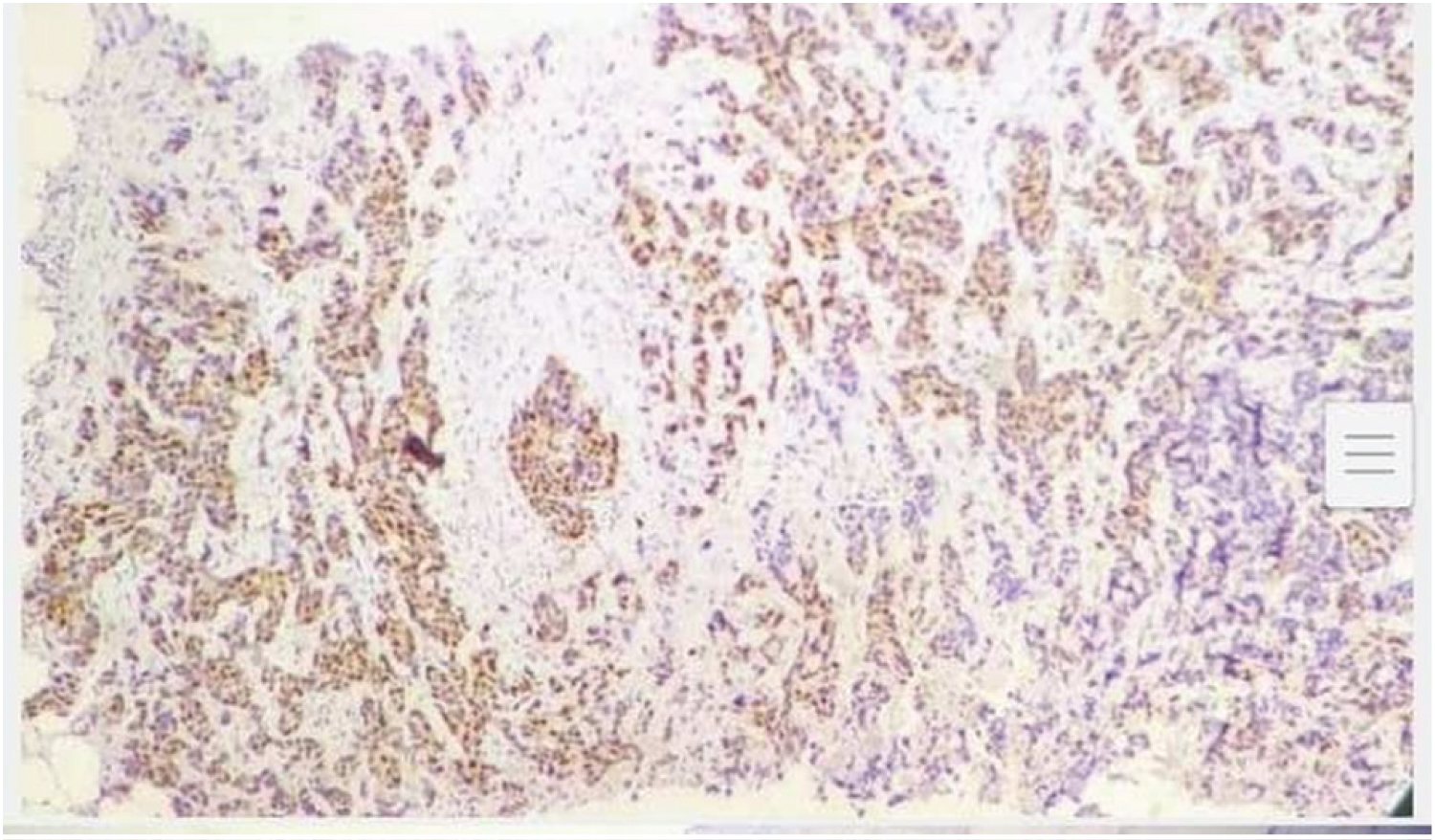 Figure 2: IHC for cyclinD1 in breast tumor 20X.
View Figure 2
Figure 2: IHC for cyclinD1 in breast tumor 20X.
View Figure 2
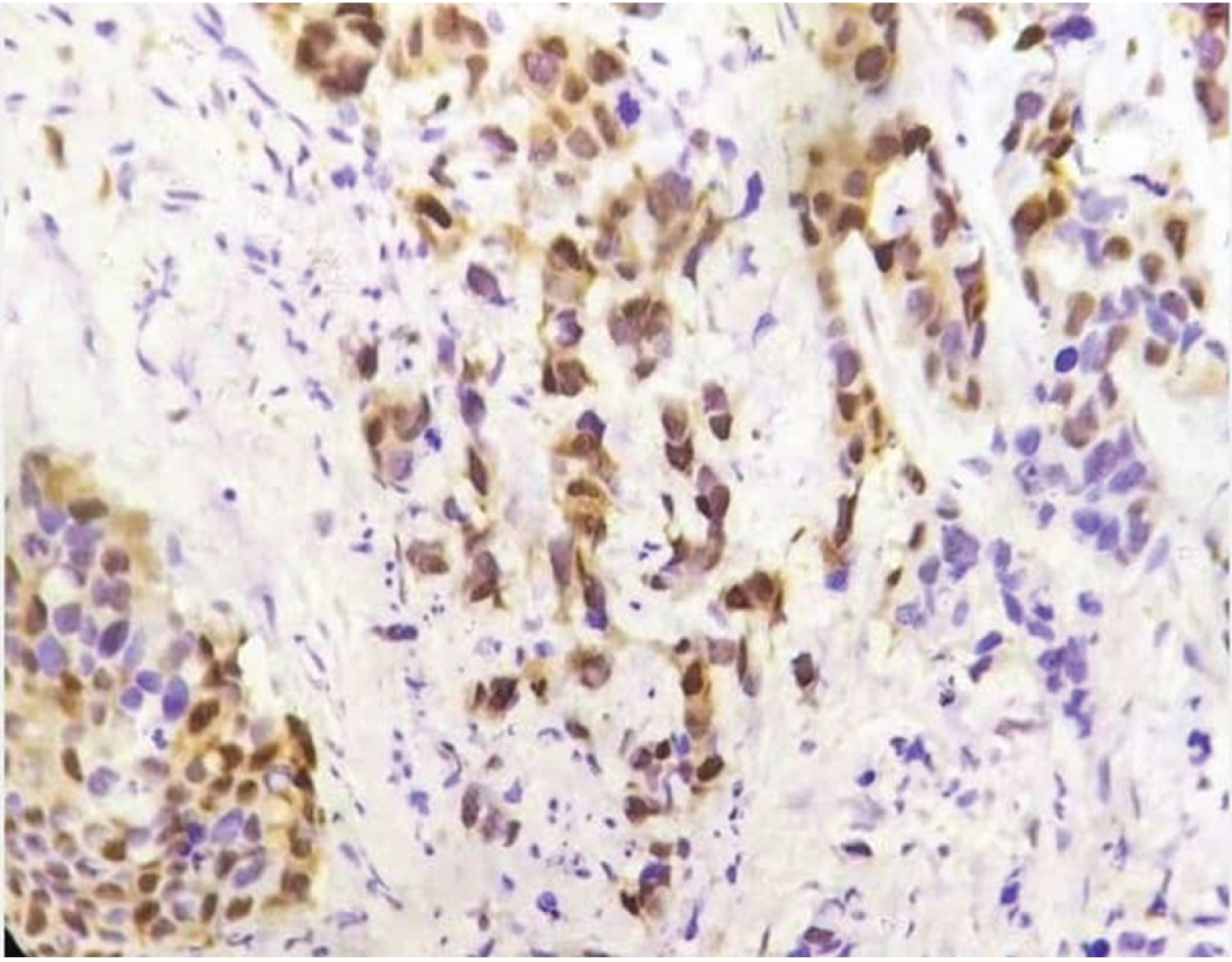 Figure 3: IHC for cyclind1 in breast cancer 40X.
View Figure 3
Figure 3: IHC for cyclind1 in breast cancer 40X.
View Figure 3
Various clinicopathological parameters are shown in the Table1. Maximum cases N = 11(36.7%) were presented in the age group 40-49 years. Minimum age of presentation was 38 years and maximum was 75 years. Mean age of presentation was 53 years. N = 21 70%) cases presented with positive expression of cyclin D1. 16 (53.3%) of cases were positive for ER, 17(56.7%) of cases were positive for PR, 4 (13.3%) cases were her2-neu positive. 13 (43.3%) of cases presented with ki67 positivity more than 10%.18 (60%) cases were MBR grade II. Our study presented the following distribution of cases Luminal A, N = 6(20%), Luminal B, N = 5(16.7%), Triple negative, N = 10(33.3%), Normal Like = 7(23.3%), Her2/neu enriched = 2(6.7%). N = 4 (40%) of triple negative molecular subtype were positive for the cyclin D1. Cyclin D1 positivity showed strong correlation with ER, PR, Her2-neu and Ki67 status (Table2). However, no other significant correlation was found with other factors.
Table 1: Correlation of clinical parameters with cyclin D1. View Table 1
Table 2: Correlation of Cyclin D1 expression with clinicopathological parameters View Table 2
CCND1 is the oncogene which is amplified in various cancers like breast, colon, lymphoma, melanoma, and parathyroid carcinoma [7]. CyclinD1 protein expression occurs even in the relative absence of the respective gene expression [8]. Studies show amplification of the CCND1 gene in 10-20% cases, however, the expression is found in 30-80% of the breast cancer [9]. This study was done to determine the correlation between the expression of cyclin D1 with other clinic pathological parameters.
CyclinD1 is necessary for the normal development of the mammary glands. Besides its role in the cell cycle, it also modulates various regulatory molecules. It has a role in the repression of the STAT3 molecule which in turn causes loss of its anti-apoptotic activities and hence causes cellular proliferation [10]. CyclinD1 is also an intermediate in NFkb related pathway [11]. CyclinD1 has a complex relationship with ER. It can directly activate the ER and thus inducing its proliferating effects. Our study shows a strong correlation of CyclinD1 with ER and PR. The same has been depicted in other studies [12]. 53.3% of tumors were positive with both cyclinD1 and ER and PR. Ahlin and co-workers demonstrated in a cohort of 364 patients of breast carcinoma that immunohistochemical positivity of Cyclin D1 is associated with increased breast cancer-related deaths in ER-positive patients even when adjusted to size and grade [13].
There was a strong correlation with Her2/neu with (p = 0.035), however many other studies showed no correlation with this marker [14]. While others presented with significant correlation. Further studies are required to solve this discrepancy.
Our study presented with the strong association of CyclinD1 and Ki67 with (p = 0.020). As the cyclind1 is a regulator of cell growth and promotes cellular proliferation. This association is expected. The same has been demonstrated in many other studies [15].
The majority of the cyclinD1 positive cases were of lower grade in our study. This reverse association with grade has been demonstrated in any other studies like Mohammadizadeh F et al and Pranoti Vitthalrao Lengare et al. [16].
A strong association of CyclinD1 was found with the molecular classification of the breast. All the luminal A and normal-like tumors in our study were positive for CyclinD1. While 80% of luminal B tumors were positive for CyclinD1 in contrast with only 40% of triple- negative tumors were positive for cyclinD1.
No association was found with age, grade, lymph node status, and metastasis.
Reporting by expert oncopathologist and following standard reporting guidelines as per CAP protocol were strength of our study. Not doing follow-up and survival analysis were limitations our study.
With the advent of CD4/6 inhibitors like palbociclib, ribocicib, and abemacicib the role of cyclinD1 in breast carcinoma and potent therapeutic effects of these agents is under research [17]. More data and trails will improve our understanding.