The objective of this review is to introduce significant research findings mainly on cytokine responses to exercise. First, some basic background information on cytokines is provided. Then, some of our data according to exercise modes and key experimental research on the factors affecting cytokine responses to exercise are explained. Furthermore, the mechanisms and modulations of the cytokine responses are described to understand stress and inflammatory reactions and their prevention/recovery.
Exhaustion, Cytokine, Chemokine, Systemic inflammation, Muscle damage, Anti-inflammatory effect of exercise
Cytokines are important intercellular signaling molecules that regulate inflammation and immune responses [1]. They are produced by a variety of cells and are classified into several different categories based on their bioactivity. Physiologically, cytokines act locally in an autocrine or paracrine manner at very low concentrations. These actions are in contrast to hormones, which exert their influence through the systemic circulation on target organs. However, pathologically, cytokines are released systemically in response to a variety of serious insults such as systemic infections, severe trauma, burn injury, and acute myocardial infarction. Therefore, the increase in the circulating concentration of cytokines (hypercytokinemia) is considered abnormal and is collectively designated as a Systemic Inflammatory Response Syndrome (SIRS) clinically [1-4].
As for the classification of cytokines [1,1], there are pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, which induce acute inflammation. Immunomodulatory cytokines such as IL-2, interferon (IFN)-γ and IL-12p70 are produced to induce cellular immunity as protection against pathogens such as viruses and intracellular bacteria. Figure 1 shows the cytokine balance based on helper T (Th) cells, and the effects of exhaustive exercise on the component cytokines are described later (Figure 1) [5]. There are also anti-inflammatory cytokines such as IL-1 receptor antagonist (IL-1ra), IL-4, IL-6, IL-10 and IL-12p40, which block action and production of other pro-inflammatory and immunomodulatory cytokines. Here, IL-6 is classified as a multi-functional cytokine, because it can act as both a pro- and an anti-inflammatory cytokine, depending on the progression of inflammation. Colony-stimulating factors are involved in hematopoiesis, mobilization and activation of neutrophils and monocytes. Chemokines are chemotactic cytokines that regulate tissue infiltration of leukocytes.
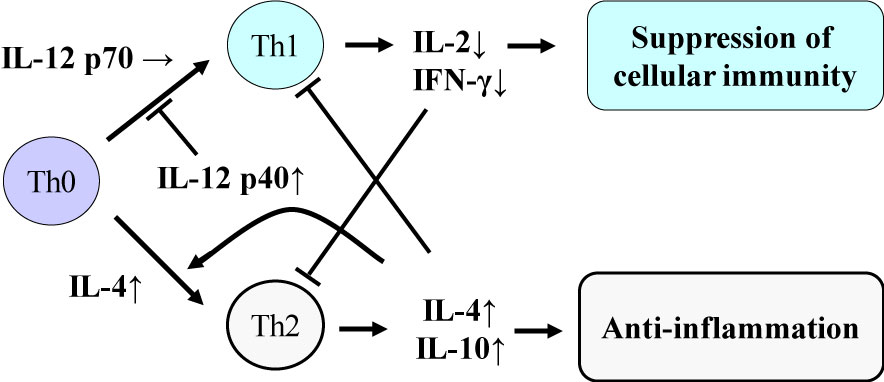 Figure 1: Cytokine balance based on helper T (Th) cells. Suzuki K, et al. [2,5]. View Figure 1
Figure 1: Cytokine balance based on helper T (Th) cells. Suzuki K, et al. [2,5]. View Figure 1
This article firstly introduces findings on the effects of most exhaustive exercise conditions, a maximal intensity exercise within 30 min, and a long duration race such as marathon, duathlon and triathlon on cytokine release in observational studies. Then, some of the main experimental research concerning the impacts on the body and the underlying mechanisms of endurance exercise-related phenomena are highlighted. Besides, our findings of cytokine dynamics following eccentric exercise in relation with muscle damage were paradoxically strengthened and the prevention/modifications are described. Past comprehensive and related reviews on cytokine response to exercise are referred to elsewhere [2,5-10].
In the mid-1990s, we had investigated the effects of exercise on immune perturbations such as neutrophil mobilization and priming following exhaustive exercise and the underlying mechanisms [11-13]. As for cytokines, at first, we measured serum concentrations of TNF-α, IL-1β and IFN-γ before and after maximal exercise on a treadmill and 1 h after exercise [13]. Although we observed exercise-elicited leukocytosis due to increases in large granular lymphocytes, monocytes and neutrophils together with enhanced neutrophil pseudopod projection and the capacity to produce Reactive Oxygen Species (ROS) [11], we could not detect the above cytokines at least up to 1 h after exercise [13]. It might be partly because the sensitivity of the Enzyme-Linked Immunosorbent Assays (ELISA) was low at that time, and it was later found that these cytokines are not easily increased in the circulation after short-time maximal exercise as compared to long endurance exercise [2,5,6,10].
In spite of the disappointed results even using expensive ELISA kits, we tried once again to investigate the time course of changes until 2 h after maximal exercise in a broader spectrum of cytokines in plasma and urine as well as employing more sensitive ELISA [14]. As a result, we found that plasma IL-1β was significantly elevated 2 h after exercise by two-fold. In urine, IL-1ra increased 15.5 fold 1 h after exercise, and TNF-α 5 fold 2 h after exercise. Plasma and urine IL-4 levels were significantly elevated in parallel only 2 h after exercise by 7 fold. Plasma Granulocyte Colony-Stimulating Factor (G-CSF) was significantly elevated immediately after exercise, which remained until 2 h after exercise. Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) in plasma was temporarily elevated immediately after exercise, and the urine level was also elevated 1 h later, indicating a temporal secretion into the circulation and subsequent excretion into urine.
These data suggested that some cytokines were rapidly excreted from the circulation and that cytokine in plasma might only reflect a minor portion of the entire amount produced in the body. Also, it was suggested that exercise stress promoted the release of cytokine antagonist and anti-inflammatory cytokines to prevent systemic inflammation. Additionally, early increments of G-CSF and GM-CSF suggest the involvement of mobilization and priming of leukocytes, which were partly demonstrated later [2,15-17]. These changes indicated that each cytokine dynamics and impacts on the body might be quite different [2,6,9,10].
Our original paper submission on marathon data was rejected again and again, and it took several years to be published finally in 2000 [18]; it was certain that the results were no changes in plasma pro-inflammatory cytokines even after a competitive full marathon race, and immunomodulatory cytokine IL-2 decreased after exercise. However, anti-inflammatory cytokines such as IL-1ra, IL-6 and IL-10 increased dramatically after the race. These changes might result in immunosuppression after exhaustive exercise [2,5,9,18]. On the other hand, colony-stimulating factors and chemokines increase significantly, which might induce mobilization and activation of neutrophils and monocytes, resulting in inflammation [18,19].
Particularly, IL-6 increased more than 100 fold following a marathon race and was correlated with changes in IL-10 [2,7,18,19], the most potent immunosuppressive cytokine. IL-6 was also correlated with neutrophil count, whereas neutrophil mobilization was correlated with changes in the muscle damage markers, Creatine Kinase (CK) and myoglobin [20]. Interestingly, IL-6 response was also correlated with increases in free fatty acids [5], which are an important energy substrate during endurance exercise. Another interesting observation from our research was that IL-6 responses were inversely related to individual race time (Figure 2) [5]. That is, IL-6 was highest in those athletes who run fastest. This observation might be related to the effects of IL-6 on the mobilization of free fatty acids [21-23]. Although IL-6 has been classified as a multi-functional cytokine, it might perform several different roles during exercise. Also, anti-inflammatory effects of IL-10 might be induced for suppression of ROS production by neutrophils and monocytes [19]. Furthermore, IL-1ra and IL-12p40 might work as antagonists of exercise-induced inflammation [2,5].
 Figure 2: IL-6 response and marathon race time. Suzuki K, et al. [5]. View Figure 2
Figure 2: IL-6 response and marathon race time. Suzuki K, et al. [5]. View Figure 2
Concerning triathlon and duathlon races [24-28], muscle damage and inflammation were more pronounced, and acute kidney injury was also observed [24,27]. On the other hand, time course changes in inflammatory markers were examined a longer duration race and the recovery and revealed that the triathlete recovered almost completely within approximately one week using inflammatory markers [28]. However, typical inflammatory markers such as C-Reactive Protein (CRP) were not sensitive enough in case of exercise, but some cytokine responses to exercise were more exaggerated in urine [2,26]. As such, experimental research was needed for further characterization of each cytokine dynamics and their roles in the exercise-induced organ damage and inflammation.
At first, we have extended the study on repeated bouts effects of exercise in relation with hormonal changes to analyze adaptation mechanisms regulating systemic inflammatory responses of the stressed body [20]; endurance exercise induced peripheral neutrophilia and neutrophil priming for ROS production. Plasma IL-6 rose significantly and was closely correlated with the neutrophil responses, which were also correlated with muscle damage markers. These exercise-induced responses were strongest on the first day, but the magnitude gradually decreased with progressive daily exercise, whereas catecholamine responses to exercise sessions gradually rose, possibly suppressing neutrophil priming and may affect local tissue damage of susceptible organs [15,16,20].
Professor Pedersen's research group in Copenhagen, Denmark, has demonstrated that contracting muscle produces IL-6 and releases it into the circulation depending on the energy demand, and IL-6 mobilizes energy substrates in a similar manner to stress hormones [29]. Therefore, IL-6 is considered to support energy supply and endurance performance and is therefore referred to as a myokine [7]. However, it was unclear whether IL-6 is released into the circulation in response to exercise-induced muscle damage or exercise intensity [2,7,20,29].
Dr. Peake from the University of Queensland in Brisbane, Australia, investigated the effects of exercise intensity and muscle damage on neutrophil activities [30]. I assisted in his work by measuring changes in several cytokines that regulate neutrophil activity during exercise [30-32]. He compared three exercise conditions using 10 male endurance-trained athletes. The first condition was moderate-intensity, level treadmill running at 60% VO2 max for 60 minutes, the second conditions was high-intensity, level treadmill running at 85% VO2 max for 60 minutes [30,31], and the last was moderate-intensity downhill running at 60% VO2 max for 60 min [32]. Figure 3 shows the changes in myoglobin, a muscle damage marker. Downhill running caused marked increase in myoglobin, indicating that this protocol caused more muscle damage than moderate- and high-intensity level running (Figure 3). However, IL-6 response was greater after high-intensity level running compared with moderate-intensity level running and downhill running, indicating that IL-6 response depends on exercise intensity rather than muscle damage (Figure 4). Also, IL-1ra increased significantly after high-intensity running, but not after downhill running (Figure 5). IL-10 also increased only after high-intensity running (Figure 6). These results suggest that systemic release of IL-6, IL-1ra and IL-10 depends more on exercise intensity than muscle damage [2,8-10]. Thus, it was considered that cytokine responses reflected stress reactions of the body.
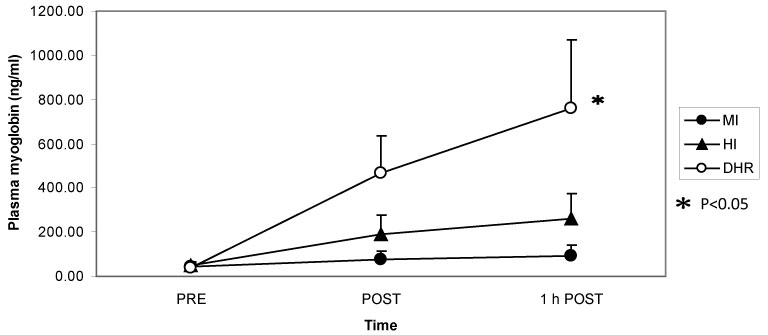 Figure 3: Myoglobin increased after downhill running (DHR) compared with moderate-intensity (MI) and high-intensity (HI) running. Peake J, et al. [30,31]. View Figure 3
Figure 3: Myoglobin increased after downhill running (DHR) compared with moderate-intensity (MI) and high-intensity (HI) running. Peake J, et al. [30,31]. View Figure 3
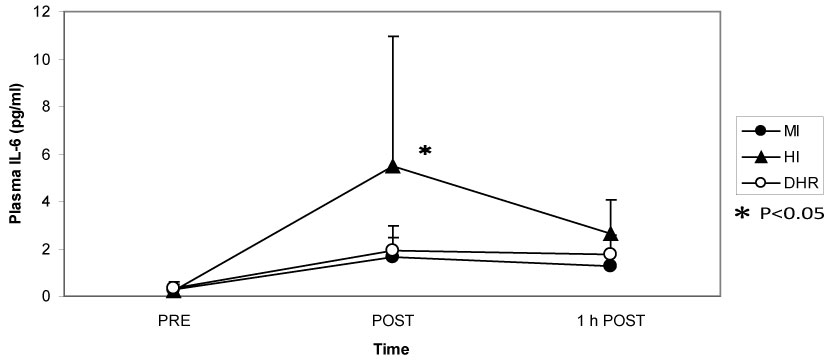 Figure 4: IL-6 increased more during high-intensity (HI) running than during moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 4
Figure 4: IL-6 increased more during high-intensity (HI) running than during moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 4
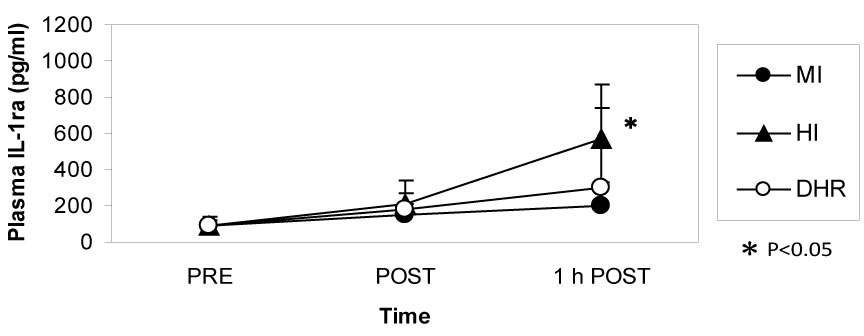 Figure 5: IL-1ra increased after high-intensity (HI) running but not after moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 5
Figure 5: IL-1ra increased after high-intensity (HI) running but not after moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 5
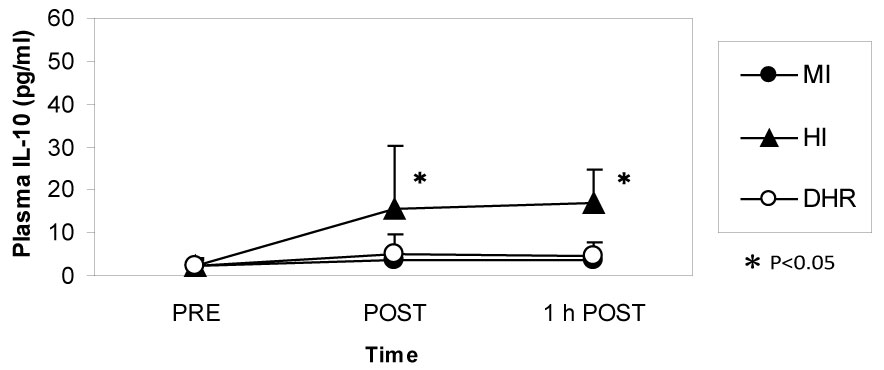 Figure 6: IL-10 increased during high-intensity (HI) running but not during moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 6
Figure 6: IL-10 increased during high-intensity (HI) running but not during moderate-intensity (MI) and downhill running (DHR). Peake J, et al. [30,31]. View Figure 6
Although it was initially proposed that IL-6 is released into the circulation in response to exercise-induced muscle damage, there was little definite evidence to support this concept. To further investigate whether there is a relationship between muscle damage and cytokines, we examined the effects of eccentric exercise which causes local muscle damage, but minimal metabolic stress with the joint study of Professor Nosaka's research group in Perth, Australia [33-35]. Ten untrained male university student repeated eccentric lengthening contraction of the elbow flexors to induce muscle damage and inflammation. Using a dumbbell, eccentric lengthening contractions were performed, and the same protocol was repeated one month later as the second bout to explore the mechanisms of the muscle adaptation. We measured muscle damage markers, cytokines in plasma and urine, and related markers of inflammation. In response to the first bout, inflammatory signs such as muscle pain and swelling, and decreases in range of motion and muscle strength occurred (Figure 7). Muscle damage markers, creatine kinase and myoglobin, also increased dramatically after the first bout (Figure 8). In contrast, these changes were much smaller after the second bout, which indicates that muscle adaptation occurred between the two bouts of exercise. In contrast with these marked changes in markers of muscle damage, there were only minor changes in plasma inflammatory mediators [33-35].
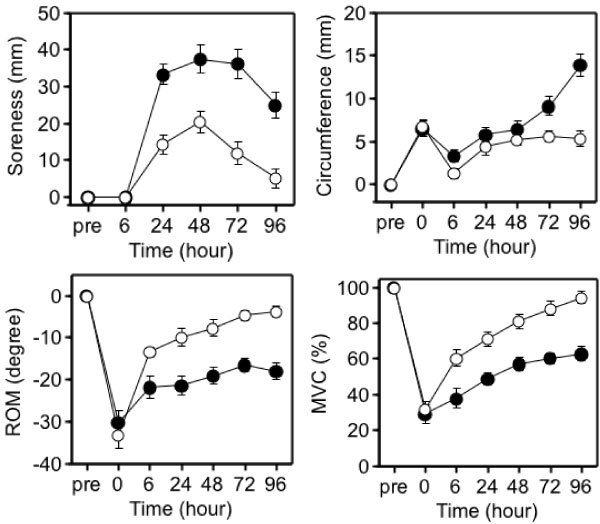 Figure 7: Responses of the elbow flexors to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
Figure 7: Responses of the elbow flexors to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
ROM: range of motion; MVC: maximal voluntary contraction. View Figure 7
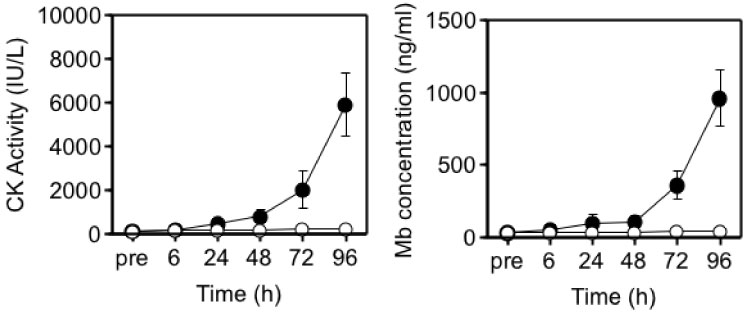 Figure 8: Responses of blood parameters to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
Figure 8: Responses of blood parameters to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
CK: creatine kinase; Mb: myoglobin. View Figure 8
We measured renal damage markers and urine cytokine levels as well to check the possibility that plasma cytokines are excreted rapidly from the kidneys into urine. However, no significant differences were observed in renal damage markers in spite of severe muscle damage like rhabdomyolysis, and renal clearance was not affected by the local eccentric exercise and muscle damage. Interestingly, we observed that there were significantly higher and more rapid increases in anti-inflammatory cytokines such as IL-1ra and IL-6 in the second bout compared with the first bout (Figure 9). In summary, although there was severe muscle damage after the eccentric exercise, the changes in cytokines were quite minor, and considerably smaller than that following endurance exercise. Nevertheless, there was induction of anti-inflammatory cytokines in the second bout, which might help restrict muscle damage.
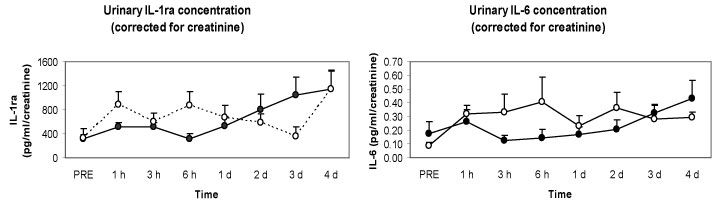 Figure 9: Responses of urinary cytokines to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
Figure 9: Responses of urinary cytokines to the 1st (●) versus 2nd (○) bouts (4 weeks apart) [33].
IL-1ra: interleukin 1 receptor antagonist; IL-6: interleukin 6. View Figure 9
We had also evidence that plasma and urine cytokine levels did not change following local eccentric exercise using a calf raise model as well [36-38]. Even though we discovered a novel marker of muscle damage in urine, N-terminal fragment of titin [38], cytokine changes were not detected even using shotgun proteomics (Figure 10). These findings indicated that muscle damage is not the trigger of the systemic cytokine release.
 Figure 10: Shotgun proteomics using urine after eccentric exercise [38]. View Figure 10
Figure 10: Shotgun proteomics using urine after eccentric exercise [38]. View Figure 10
Energy supply during exercise is important for the endurance performance. In a previous study, Professor Nieman, at Appalachian State University, USA, investigated the effects of carbohydrate drink on plasma cytokine levels and muscle cytokine gene expression following 3 h of treadmill running at 70% VO2 max [39]. They demonstrated that carbohydrate drink intake reduced plasma IL-6 concentrations and muscle IL-6 gene expression. Plasma IL-1ra and IL-10 were also reduced by carbohydrate drink. These findings suggest that carbohydrate ingestion before and during exercise is useful to prevent not only a decline in endurance performance by sparing muscle glycogen stores, but also exercise-induced muscle damage.
However, some studies reported that carbohydrate ingestion after eccentric exercise enhanced the increase in circulating IL-6 levels, gene expression of IL-8 and MCP-1 in skeletal muscle, and muscle soreness [40]. Although carbohydrate ingestion after endurance exercise is recommended to enhance muscle glycogen synthesis, these findings suggest that post-exercise carbohydrate ingestion may promote muscle inflammation via the promotion of neutrophil and macrophage infiltration in skeletal muscle.
We examined the effects of ingestion of different amounts of carbohydrate after endurance exercise on circulating cytokine levels, neutrophil count, and the markers of neutrophil activation and muscle damage, and revealed that carbohydrate ingestion after endurance exercise did not enhance the exercise-induced increase in above variables, regardless of the amount of carbohydrate ingested [41]. Furthermore, the ingestion of a high amount of carbohydrate maintained high plasma glucose and insulin concentrations during the recovery phase when compared to the ingestion of a low amount of carbohydrate. These findings suggest that the ingestion of a high amount of carbohydrate after endurance exercise provides a favorable condition for the recovery from endurance exercise without an increase in inflammatory responses and markers of muscle damage.
Heat stress might attenuate the effects of carbohydrate on immunoendocrine responses to exercise by increasing endogenous glucose production and reducing the rate of exogenous carbohydrate oxidation. We compared the efficacy of carbohydrate consumption on immune responses to exercise in temperate vs. hot conditions. Ten male cyclists exercised on 2 separate occasions in temperate (18.1 ± 0.4 ℃, 58% ± 8% relative humidity) and on another 2 occasions in hot conditions (32.2 ± 0.7 ℃, 55% ± 2% relative humidity). On each occasion, the cyclists exercised in a fed state for 90 min at ~ 60% VO2 max and then completed a 16.1-km time trial. Every 15 min during the first 90 min of exercise, they consumed 0.24 g/kg body mass of a carbohydrate or placebo gel. Neutrophil counts increased during exercise in all trials and were significantly lower after the carbohydrate than after the placebo trials in 32 ℃. The circulating IL-6, IL-8, IL-10, G-CSF, myeloperoxidase and calprotectin also increased during exercise in all trials but did not differ significantly between the carbohydrate and placebo trials. Carbohydrate ingestion attenuated neutrophil counts during exercise in hot conditions, whereas it had no effect on any other immune variables in either temperate or hot conditions [42,43].
Also, we conducted a study on cytokine responses to exercise in warm and cool environments in a joint study with Professor Kim at Keimyung University in Korea [44,45]. The aims of this study was to investigate whether a cool environment affects cytokine responses to exercise. Since skating is very popular in Korea, short-track skaters who are adapted to exercise in the cold and inline skaters who are adapted to exercise in warm conditions were recruited for the study. Both groups of athletes exercised in either cool or warm conditions on separate days. In the first protocol, inline skaters and short-track skaters stayed at room temperature for 60 min before cycling at 65% VO2 max for 60 min, and then recovered for 2 h. Under these conditions, the short-track skaters who were adapted to exercising in cool conditions were exposed to unaccustomed stress. In the second protocol, the two groups of subjects stayed in a cool environment for 60 min before cycling at 65% VO2 max for 60 min in the cool environment, and then recovered at room temperature for 2 h. Under these conditions, the inline skaters who are adapted to exercising in warm conditions were exposed to unaccustomed stress. Among 4 conditions, when the short-track skaters exercised in unaccustomed warm conditions (SW), IL-6 increased dramatically, whereas for these athletes, this response was reduced when exercising in familiar cool conditions (Figure 11). IL-1ra (Figure 12) and IL-10 (Figure 13) also shows the highest response in SW. Cortisol was elevated immediately after exercise, but the most dramatic changes were observed in SW (Figure 14). Cortisol responses were smaller following exercise in the cool in both groups. As for the muscle damage marker, the pattern of changes was different in that muscle damage was greater for the inline skaters when they exercised under unaccustomed cool conditions (Figure 15). In summary, this study demonstrated that cytokine responses to exercise depended on stress and body temperature elevation, independent of muscle damage. The short-track skaters showed marked increases in plasma cytokines in the warm, suggesting that cool environments did not cause cytokine and stress hormone responses to exercise.
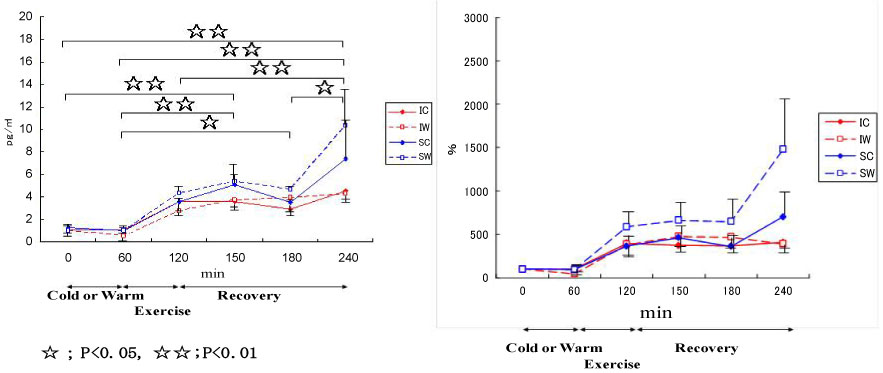 Figure 11: Responses of IL-6 to exercise in cool and warm environments [44].
Figure 11: Responses of IL-6 to exercise in cool and warm environments [44].
I: inline skaters; S: short-track skaters; C: cool; W: warm. View Figure 11
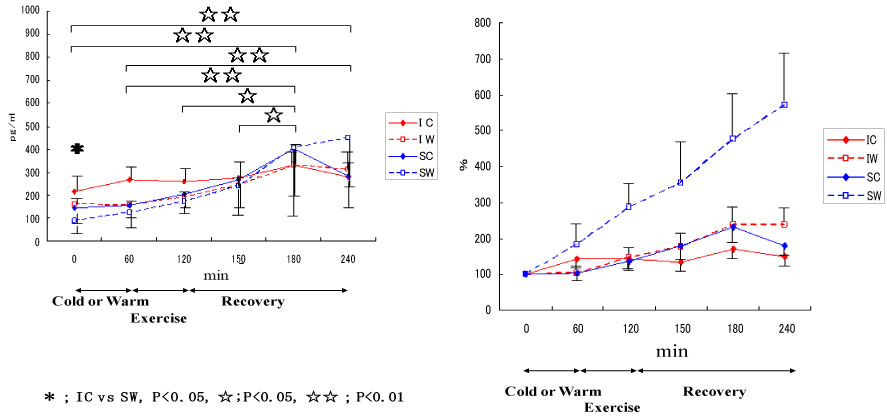 Figure 12: Responses of IL-1ra to exercise in cool and warm environments [44].
Figure 12: Responses of IL-1ra to exercise in cool and warm environments [44].
I: inline skaters; S: short-track skaters; C: cool; W: warm. View Figure 12
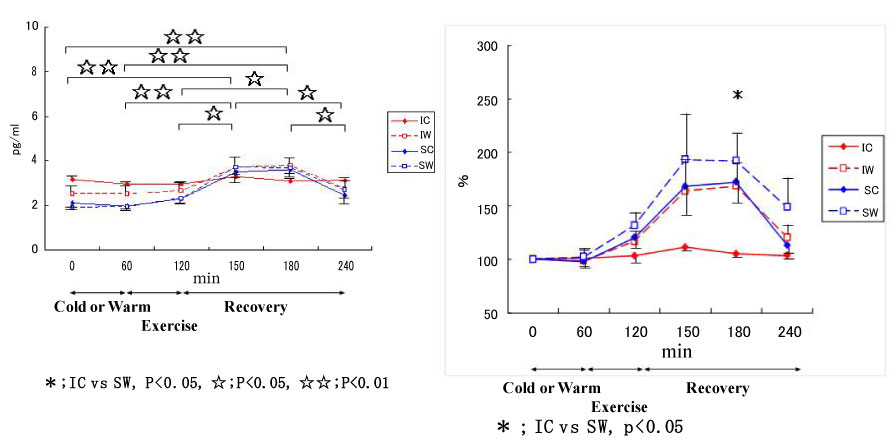 Figure 13: Responses of IL-10 to exercise in cool and warm environments [44].
Figure 13: Responses of IL-10 to exercise in cool and warm environments [44].
I: inline skaters; S: short-track skaters; C: cool; W: warm. View Figure 13
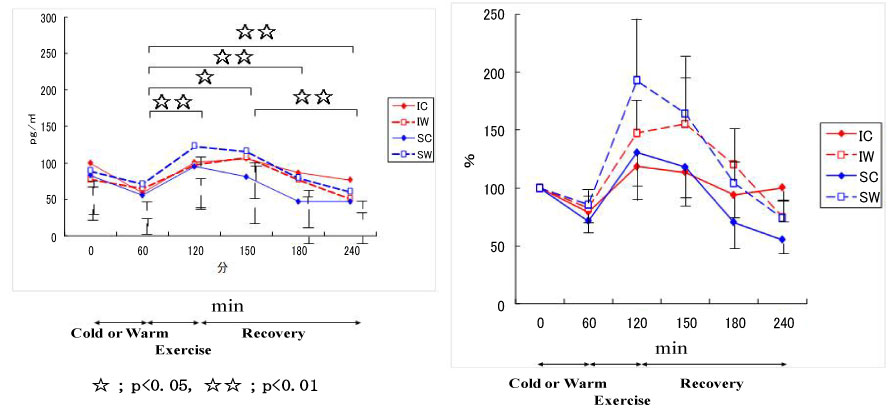 Figure 14: Responses of cortisol to exercise in cool and warm environments [44].
Figure 14: Responses of cortisol to exercise in cool and warm environments [44].
I: inline skaters; S: short-track skaters; C: cool; W: warm. View Figure 14
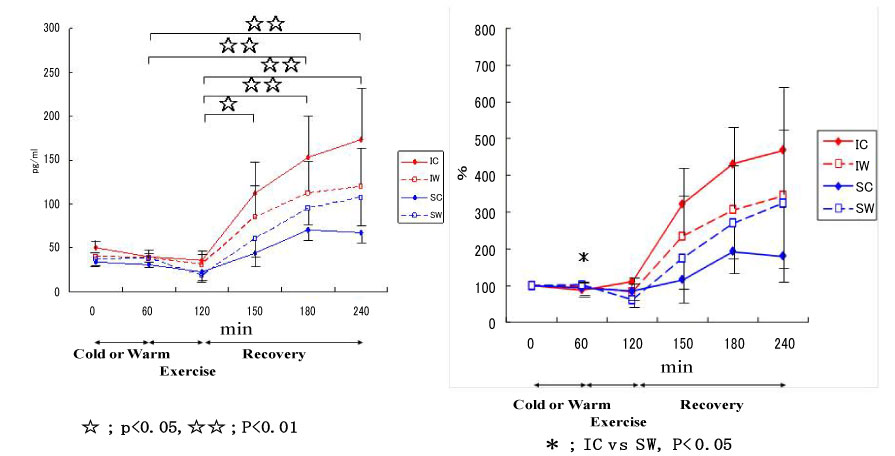 Figure 15: Responses of myoglobin to exercise in cool and warm environments [44].
Figure 15: Responses of myoglobin to exercise in cool and warm environments [44].
I: inline skaters; S: short-track skaters; C: cool; W: warm. View Figure 15
Although pre-exercise cooling can effectively attenuate systemic inflammatory response to exhaustive exercise [3,6], post-exercise cooling may not have significant effects [44-46]. During the day, evening exercise induces more marked IL-6 release than morning exercise [21], and it is recommended to exercise in cooler environments in the morning.
As for fluid supplementation, we conducted a study to evaluate the effectiveness of a hypotonic carbohydrate drink that is easy to drink and is rapidly absorbed. That is because fluid supplementation in combination with carbohydrate ingestion also prevents dehydration and heat stroke [3]. Male cyclists completed three cycling trials at 60% VO2 max for 90 min in hot conditions [47]. Three different drinks were ingested ad libitum during 90-min cycling; isotonic drink, hypotonic drink and water as the placebo control. As for the fluid intake during 90-minute exercise, the subjects consumed more of the hypotonic drink than water. Carbohydrate drinks were effective to minimize dehydration, which was also confirmed by the plasma volume changes. As for the metabolic responses, the isotonic drink caused hyperglycemia, whereas the hypotonic drink kept the glucose level constant. Exercise induced an increase in free fatty acids, but the hypotonic drink was the most effective to spare this mobilization in the recovery period. Neutrophil responses were reduced post exercise by carbohydrate ingestion. Plasma IL-6 response was reduced by the hypotonic drink. There were no differences in body temperature, heart rate, lactate, osmolality, muscle damage markers or cytokines other than IL-6. In summary of this drink study, carbohydrate significantly reduced free fatty acids and leukocyte responses to endurance exercise in the heat, but the isotonic glucose drink caused hyperglycemia post exercise. The hypotonic drink effectively attenuated IL-6 responses without inducing IL-1ra and IL-10 responses. These results suggest that the hypotonic drink is more appropriate to prevent dehydration and immune changes compared with the isotonic drink and water.
We have also investigated the effects of ingredients of sport drinks to prevent inflammation [41,47-49], but an appropriate regime remains to be examined at present. Especially, for women, intensive exercise in the menstruation phase of the menstruation cycle increases systemic inflammation [50], and energy and fluid supplementation during exercise must be considered depending on the phase [51]. From a nutraceutical perspective, curcumin ingestion has reduced muscle inflammation after downhill running in a mouse model of exercise-induced muscle damage and oxidative stress in humans [16,52,53], but the evidence is not sufficient for other antioxidant and/or anti-inflammatory substances for the prevention of muscle damage and inflammation [54-60]. Intestinal permeability increases following exercise, and endotoxemia occurs, which induces systemic inflammation [3,61-63]. Therefore, other countermeasures such as intake of some functional foods for gut barrier protection, better bioavailability, and distribution [6,64], and appropriate immune responsiveness with reducing inflammation [65-67], should be examined in future studies.
Cytokines featured at the forefront of biomedical research in 1990s [1] and we have examined the effects of exercise on the body and their modulations together with underlying mechanisms of action. IL-6 might enhance endurance performance, but might also induce systemic inflammation, muscle damage and immunosuppression. It is possible that appropriate countermeasures such as consuming energy and fluids, and minimizing rises in body temperature during exercise, might help to maintain endurance performance without causing harmful side effects on the body resulting from inflammation.
I have conducted this work with the help of domestic and international collaborators, and gratefully appreciate them and my laboratory team for research progress. Parts of these contents were presented in the Presidential Symposium in 13th International Society of Exercise and Immunology (ISEI) congress (Coimbra, Portugal, 2017). This work has been supported by a Grant-in-Aid for the Scientific Research (A). Also, this study was part of research activities of the Human Performance Laboratory, Organization for University Research Initiatives, Waseda University.
The author declares no conflicts of interest.