Stressful environmental conditions are ecological force in modulating adaptive responses of fish populations in aquatic ecosystems with a large number of biochemical and physiological effects associated with increased fluxes of oxyradicals. This study was aimed to examine the impacts of oxidative stress and histological changes in organs of Chrysichthys nigrodigitatus from Ologe and Badagry lagoons. Biomarkers such as biochemical markers [malondialdehyde (MDA) and superoxide dismutase (SOD) and catalase (CAT) and glutathione (GSH)] and histological marker were analysed in C. nigrodigitatus using standard protocols. The biochemical response of C. nigrodigitatus indicated that there was significant increase (p < 0.05) in the activities of MDA and SOD, as well as CAT and GSH in the gill, liver and muscle of the fish samples of both lagoons. The CAT activities in the muscle negatively correlated with MDA in gill (p < 0.05, r = -0.848) in Ologe lagoon. The histological changes in the gill exhibited mild epithelial lifting, fusion of secondary lamellae; inter-lamella hyperplasia, desquamation and necrosis of secondary lamellae. Furthermore, the hepatic tissue showed disseminated moderate microvesicular vacuolation of hepatocytes, moderate vascular congestion and mild presence of eosinophilic fluid within the interstitial. The renal tissue also showed mild hemorrhagic lesion, mild moderate ballooning degeneration of renal tubules and thickened blood vessel. The study provides baseline information that can be useful for effective biomonitoring and future comparative studies of oxidative stress and histomorphological changes in aquatic biota from polluted coastal ecosystems.
Biomonitoring, Biochemical responses, Histopathology, Fish, Polluted lagoons
Biomarker for biomonitoring environmental quality in aquatic ecosystem had raised a great deal and promising tool of interest caused by its economical method, early warning signal, adequate in the assessment of overall toxicities of complex mixtures and measurement precision [1]. Industrial effluents and other admixtures of toxic substances are discharged in our coastal environment which can cause changes in the physical, chemical and biological characteristics of the aquatic ecosystem thereby creating an ecological imbalance. The released xenobiotic substances bioavailable in the aquatic system tends to bind to specific cellular structures called receptors that are localized on the cell surface or inside the cell either in its cytoplasm or on cell organelles. The binding of a xenobiotic with its receptor may induce cellular processes that have toxic or other adverse effects on the cell. Biochemical markers are measurable responses of the exposed aquatic organism to xenobiotic compounds present in the water [2].
The systematic use of aquatic organisms to evaluate changes in water quality is defined as biomonitoring. Fish are often used to monitor urban and industrial effluents as well as to assess contaminant levels of coastal environments. Aquatic organisms such as fish and molluscs serve as bioindicators of pollution, due to a great deal of response and sensitivity to changes in the aquatic environment, which plays an increasingly important role in biomonitoring of environmental pollution. Biomarker was considered as the reliable method to evaluate the biological response towards environmental risk so that preventive measures can be taken [3,4].
Biomarkers have been classified by the extent that they reflect exposure to environmental stressors, or adverse health effects from contaminant exposures [5]. A biomarker has the advantage to elucidate the stress level through bioassay on the organism at various stages from biomolecular, histologically to physiological alteration induced by environmental stressors. The underlying principle in biomarker approach is the analysis of an organism with reference to the response to persistent pollutant exposure in terms of sub-organismic (cellular, biochemical, molecular, or physiological) changes, [6]. Histopathological changes have been widely used as biomarkers in the evaluation of the health of fish exposed to contaminants [7]. Gill, kidney and liver are responsible for vital functions, such as respiration, excretion and the accumulation and biotransformation of xenobiotics in the fish. One of the great advantages of using histopathological biomarkers in environmental monitoring is that this category of biomarkers allows examining these specific target organs [8].
Aquatic organisms has complement of antioxidant enzymes which comprise antioxidant defenses, including enzymatic components such as superoxide dismutase (SOD), catalase and glutathione peroxidases) as well as small molecule antioxidants (e.g. glutathione). Since antioxidant defenses can be quantitatively altered on exposure to contaminants, this has led to their exploitation as biomarkers in the field [5,9].
In Nigeria, Doherty, et al. [10] investigated biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in some selected fishes in Lagos; Otitoloju and Olagoke [11] studied lipid peroxidation and antioxidant defense enzymes in C. gariepinus as useful biomarkers for monitoring exposure to polycyclic aromatic hydrocarbons, while Ejimadu, et al., [12] assessed cellular biomarker responses of C. nigrodigitatus in contaminated coastal ecosystems in Lagos.
Ologe and Badagry Lagoons are among the nine continuous Lagoon systems found along the coast of Nigeria. These aquatic ecosystems like other coastal waters are faced with threats from inputs of compounds which have the potential to alter biochemical processes in fishes and shell fishes. According to Akoachere, et al. [13] and Onuorah and Wim, [14], these Lagoons are under intense pressure from increasing human and industrial activities around them; ultimately serving as sink for domestic and industrial waste. However, little information is available relative to the biochemical and histopathological changes in fish species in Ologe and Badagry lagoons. Consequently, studies on fish biomarker are of great significance as they can help to evaluate organ alterations, enzymatic responses, and histological changes when exposed to polluted aquatic environment.
The Ologe and Badagry Lagoons are part of the Lagos Lagoon complex within the southwestern region of Nigeria (Figure 1). Ologe Lagoon is a freshwater body which opens into the Atlantic Ocean via the Badagry creeks and the Lagos harbour. The Badagry Lagoon which is approximately 60 km long and 3 km wide, lies between longitudes 3°0' and 3°45' E and between latitudes 6°25' and 6°30' N. It is part of a continuous system of lagoons and creeks along the coast of Nigeria from the border with the Republic of Benin to the Niger delta. Its water depth ranges from 1 m to 3 m. These lagoons meet several socio-economic needs (aquaculture, fishing, sand dredging and drainage) of the various towns and villages bordering it [15].
 Figure 1: Map of sampling stations at Ologe and Badagry Lagoons.
View Figure 1
Figure 1: Map of sampling stations at Ologe and Badagry Lagoons.
View Figure 1
Fish samples (Chrysichthys nigrodigitatus) were caught with trap basket by local fisher folks set at the bottom of the lagoons, due to the habitat of the fish species been bottom dwellers (demersal). A total of 192 fish sample were collected in triplicates from Ologe and Badagry lagoons in four ecological seasons (4 dry and 4 wet seasons). Sampling was done on the basis of proximity to anthropogenic activities as hotspots across eight (8) sampling stations.
Tissues were removed from the Chrysichthys nigrodigitatus after decapitation. The gill, liver, and muscle tissues were excised, weighed, and washed in ice-cold buffer. The minced tissues were rinsed clear of blood with cold isolation buffer and homogenized on ice in a glass Potter-Elvehjem homogenizing vessel with a motor-driven pestle. The isolation buffer contained 100 mMTris-HCl; pH of 7.20 was adjusted with HCl.
The Catalase activity was determined by measuring the decrease in absorbance at 240 nm due to the decomposition of H2O2 in a UV recording spectrophotometer [16]. The reaction mixture (3 ml) contained 0.1 ml of tissue homogenate in phosphate buffer (50 mM, pH 7.0) and 2.9 ml of 30 mM H2O2 in phosphate buffer pH 7.0. An extinction coefficient for H2O2 at 240 nm of 40.0 M-1cm-1 was used for the calculation [16]. The specific activity of catalase was expressed as moles of H2O2 reduced per minute per mg protein.
Superoxide dismutase (SOD, E.C. 1.15.1.1) activity in supernatant was determined according to Kostiuk, et al. [17]. The reaction mixture (3 ml) contained 2.95 ml 0.05 M sodium carbonate buffer pH 10.2, 0.02 ml of tissue homogenate and 0.03 ml of epinephrine, 0.005 N HCL was used to initiate the reaction. The reference cuvette contained 2.95 ml buffer, 0.03 ml of substrate (epinephrine) and 0.02 ml of water. Enzyme activity was calculated by measuring the change in absorbance at 480 nm for 5 minutes.
The reduced glutathione content of the tissue as non-enzymatic was estimated according to the method described by Jollow, et al. [18]. To the tissue homogenate, 10% TCA was added, centrifuged. 1.0 ml of supernatant was treated with 0.5 ml of Ellman's reagent (19.8 mg of 5, 5-dithiobisnitro benzoic acid (DTNB) in 100 ml of 0.1% sodium nitrate) and 3.0 ml of phosphate buffer (0.2 M, pH 8.0). The absorbance was read at 412 nm.
Malondialdehyde (MDA) an index of lipid peroxidation was determined using the method of Buege and Aust [19]. 1.0 ml of the supernatant was added to 2 ml of (1:1:1 ratio) TCA-TBA HCL reagent (thiobarbituric acid 0.37%, 0.24N HCL and 15% TCA) tricarboxylic acid-thiobarbituric acid-hydrochloric acid re-agent boiled at 100 ℃ for 15 minutes, and allowed to cool. Flocculent materials were removed by centrifuging at 3000 rpm for 10 minutes. The supernatant was removed and the absorbance read at 532 nm against a blank. MDA was calculated using the molar extinction coefficient for MDATBA- complex of 1.56 × 105 M/Cm.
Ten fish species from each lagoons were collected, anesthetized, while the organs (gill, liver and kidney) were removed. Tissues were placed in prelabeled, individual glass vials in which Bouin's fixative was added. After 24 h immersion fixation, tissues were transferred to coded glass vials containing 70% ethanol. Tissues dehydrated in concentrations of alcohol, and embedded in paraffin wax for microtome sectioning (5 µm) [20]. Sections were mounted onto slides and after hydration in ethanol series, and then stained with standard hematoxylin and eosin (H&S) stain. Ten sections of each tissue from each fish were examined by light microscope [21].
All data are presented as means ± standard error media (S.E.M). Analysis of variance (ANOVA) was set at 0.05 level of significance, Duncan multiple-range test (DMRT) was used to detect the source of the differences and Pearson correlation coefficient for biochemical parameters analysed using PAST 3.16 and SPSS 20.0 software [22].
The biochemical response (Superoxide dismutase- SOD, Catalase- CAT, Glutathione- GSH and Malondialdehyde- MDA) and histopathological changes in the organs of C. nigrodigitatus from Ologe and Badagry lagoons.
The mean highest activities of SOD significantly increased in the liver with 61.33 µmol/mg during the wet season and lowest in the muscle with 34.64 µmol/mg during the dry season at Ologe Lagoon. At Badagry lagoon, the mean highest activities of SOD also increased significantly in the muscle with 110.56 µmol/mg during the wet season and lowest in the liver with 33.88 µmol/mg in the dry season (Figure 2a, Figure 2b and Figure 2c).
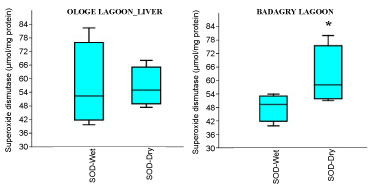 Figure 2A: Seasonal variations of Superoxide dismutase (SOD) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2A
Figure 2A: Seasonal variations of Superoxide dismutase (SOD) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2A
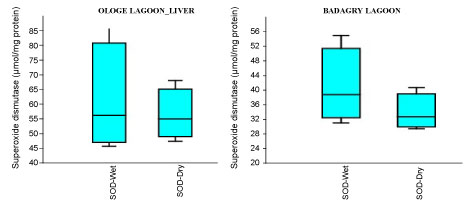 Figure 2B: Seasonal variations of Superoxide dismutase (SOD) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2B
Figure 2B: Seasonal variations of Superoxide dismutase (SOD) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2B
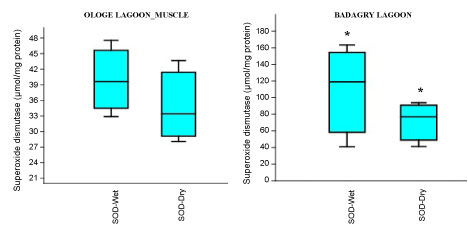 Figure 2C: Seasonal variations of Superoxide dismutase (SOD) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2C
Figure 2C: Seasonal variations of Superoxide dismutase (SOD) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 2C
Analysis of variance (ANOVA) showed that there was a significant difference (p < 0.05) in SOD activities of the gill during the dry season as well as in the muscle of C. nigrodigitatus in both wet and dry seasons at Badagry lagoon. The seasonal variations of SOD activities in the gill showed significant increase in wet season at Ologe lagoon and decreased activities in the dry season at Badagry lagoon.
The mean activities of CAT decreased significantly in the muscle with 0.51 µmol/mg during the dry season and slightly increase in the liver with 1.43 µmol/mg during the wet season at Ologe Lagoon. While, at Badagry Lagoon, the activities of CAT decreased significantly in the gill and muscle with 0.70 µmol/mg during the dry season and slightly increase in gill (0.79 µmol/mg), and liver (0.90 µmol/mg) in the wet season (Figure 3a, Figure 3b and Figure 3c). The ANOVA showed that there was a significant difference (p < 0.05) in CAT activities in the organs of C. nigrodigitatus in Ologe lagoon across the seasons.
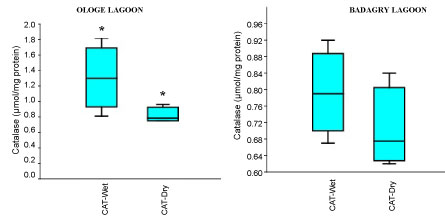 Figure 3A: Seasonal variations of Catalase (CAT) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3A
Figure 3A: Seasonal variations of Catalase (CAT) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3A
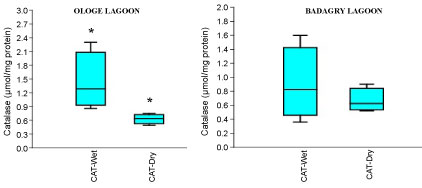 Figure 3B: Seasonal variations of Catalase (CAT) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3B
Figure 3B: Seasonal variations of Catalase (CAT) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3B
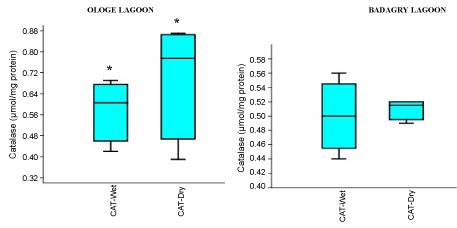 Figure 3C: Seasonal variations of Catalase (CAT) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3C
Figure 3C: Seasonal variations of Catalase (CAT) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 3C
The mean content of GSH observed highest in gill and liver with 0.15 µmol/mg during the dry season, and lowest mean GSH activities in the muscle with 0.09 µmol/mg during the wet season at Badagry Lagoon. While at Ologe Lagoon, the mean GSH content observed highest in the gill and liver with 0.14 µmol/mg during the dry season, and lowest in the gill with 0.11 µmol/mg during the wet season respectively (Figure 4a, Figure 4b and Figure 4c). ANOVA showed that there were significant differences (p < 0.05) in GSH content in liver and muscle during the dry season at Ologe lagoon. Although, there were observable increase in the GSH content in the gill and liver of the fish species at the dry season in Ologe and Badagry lagoons. This could be interpreted as the cellular adaptation response to initial oxidative stress during the dry season as the diluents of contaminants (admixture of chemical constituents) are far concentrated in the gill and liver of the fish species.
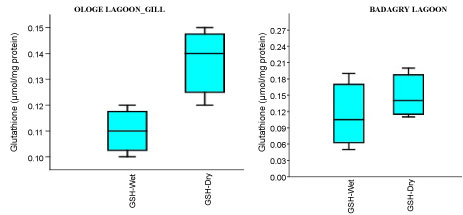 Figure 4A: Seasonal variations of reduced Glutathione (GSH) content in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4A
Figure 4A: Seasonal variations of reduced Glutathione (GSH) content in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4A
 Figure 4B: Seasonal variations of reduced Glutathione (GSH) content in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4B
Figure 4B: Seasonal variations of reduced Glutathione (GSH) content in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4B
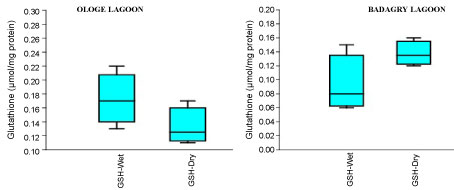 Figure 4C: Seasonal variations of reduced Glutathione (GSH) content in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4C
Figure 4C: Seasonal variations of reduced Glutathione (GSH) content in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 4C
The MDA activities observed a significant increase (p < 0.05) in the gill with mean values of 26.37 µmol/mg and 28.13 µmol/mg in the dry and wet season at Badagry Lagoon, while 16.08 µmol/mg and 26.03 µmol/mg in the dry and wet season at Ologe Lagoon respectively (Figure 5a, Figure 5b and Figure 5c). ANOVA exhibited significant differences (p < 0.05) in MDA activities in the organs of C. nigrodigitatus at Ologe lagoon across the seasons.
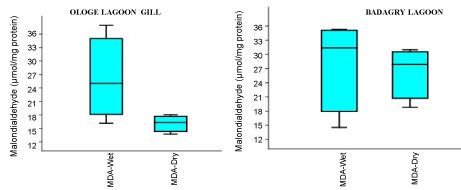 Figure 5A: Seasonal variations of Malondialdehyde (MDA) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5A
Figure 5A: Seasonal variations of Malondialdehyde (MDA) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5A
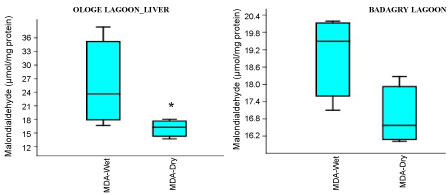 Figure 5B: Seasonal variations of Malondialdehyde (MDA) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5B
Figure 5B: Seasonal variations of Malondialdehyde (MDA) in the liver of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5B
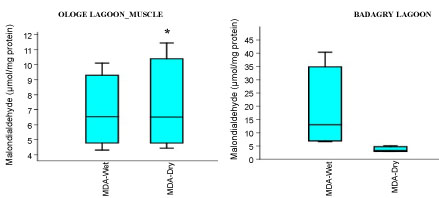 Figure 5C: Seasonal variations of Malondialdehyde (MDA) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5C
Figure 5C: Seasonal variations of Malondialdehyde (MDA) in the muscle of C. nigrodigitatus at Ologe and Badagry Lagoons.
View Figure 5C
The Pearson correlation coefficient showed that MDA activities in gill and CAT in muscle exhibited a negative significant correlation (r = -0.848, p < 0.05); SOD activities in muscle and CAT in muscle showed negative significant correlation (r = -0.824, p < 0.05) in C. nigrodigitatus in Ologe lagoon. While, the GSH activities in gill and CAT activities in liver exhibited a negative significant correlation (r = -0.847, p < 0.05); CAT activities in liver and SOD activities in muscle exhibited a positive significant correlation (r = 0.851, p < 0.05); MDA activities in muscle and CAT activities in liver showed positive significant correlation (r = 0.880, p < 0.05); while SOD activities in liver and MDA activities in muscle (r = 0.879, p < 0.05) in C. nigrodigitatus in Badagry lagoon (Table 1 and Table 2).
Table 1: Body weight changes after iv administration of different doses of DINP and other test materials in male Sprague-Dawley rats for 4 wk. View Table 1
Table 2: Absolute and relative organ weights in male Sprague-Dawley rats after repeated iv infusions of DINP and other test materials for 4 wk. View Table 2
The histopathological changes in the gill of C. nigrodigitatus from the control group showed normal arrangement pattern of primary and secondary lamellae. Varied morphological changes occurred in the gill tissue of the affected C. nigrodigitatus and the gill exhibited marked mild epithelial lifting. Thus, there was fusion at adjacent secondary lamellae as a result of hyperplasia. Inter-lamella hyperplasia, desquamation and necrosis of secondary lamellae were observed. The mild stunted growth of primary lamellae in the histological sections of gills of the C. nigrodigitatus affected with aquatic pollutants of Ologe lagoon (Plate 1, Plate 2 and Plate 3).
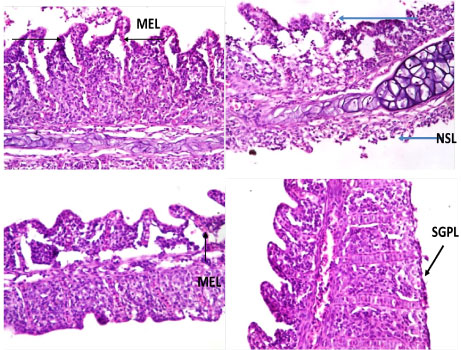 Plate 1: Photomicrographs of gill show mild stunted growth of primary lamellae (SGPL), mild epithelial lifting (MEL), fusion of secondary lamellae (FSL), inter-lamella hyperplasia, desquamation and necrosis of secondary lamellae (NSL).
Plate 1: Photomicrographs of gill show mild stunted growth of primary lamellae (SGPL), mild epithelial lifting (MEL), fusion of secondary lamellae (FSL), inter-lamella hyperplasia, desquamation and necrosis of secondary lamellae (NSL).
The histomorphology in the liver of C. nigrodigitatus exposed to aquatic pollutant from Ologe and Badagry lagoons showed diffuse changes in hepatic tissue with disseminated moderate microvesicular vacuolation of hepatocytes, moderate vascular congestion and mild presence of eosinophilic fluid within the interstitial.
View Plate 1
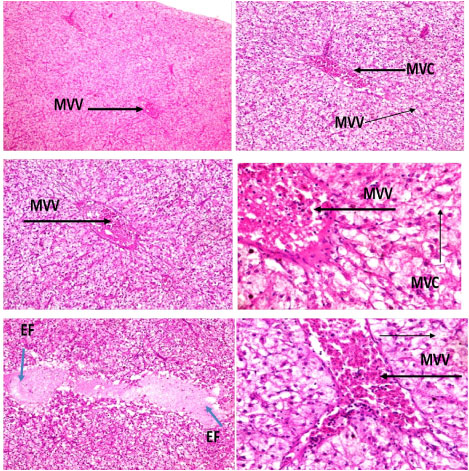 Plate 2: Photomicrographs of hepatic tissue show disseminated moderate microvesicular vacuolation (MVV) of hepatocytes, moderate vascular congestion (MVC) and mild presence of eosinophilic fluid (EF) within the interstitial.
Plate 2: Photomicrographs of hepatic tissue show disseminated moderate microvesicular vacuolation (MVV) of hepatocytes, moderate vascular congestion (MVC) and mild presence of eosinophilic fluid (EF) within the interstitial.
Lesions were observed in the histological studies of affected kidney of C. nigrodigitatus. Renal tissue showed mild haemorrhagic lesion, mild moderate ballooning degeneration of renal tubules and thickened blood vessel.
View Plate 2
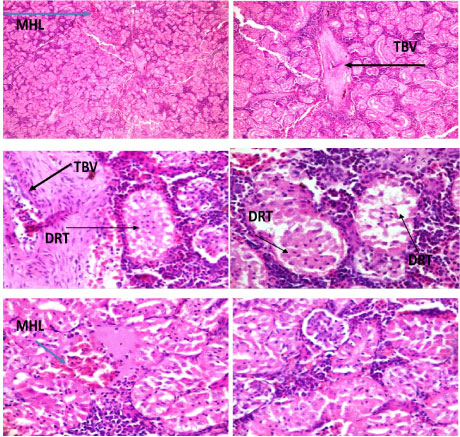 Plate 3: Photomicrographs of renal tissue show mild haemorrhagic lesion (MHL), mild moderate ballooning degeneration of renal tubules (DRT) and thickened blood vessel (TBV).
View Plate 3
Plate 3: Photomicrographs of renal tissue show mild haemorrhagic lesion (MHL), mild moderate ballooning degeneration of renal tubules (DRT) and thickened blood vessel (TBV).
View Plate 3
The aquatic ecosystems are extensively contaminated with admixtures of chemical compounds including heavy metals, persistent organic pollutants released from industrial and other anthropogenic sources causing potent harm on the ecological equilibrium of the aquatic environment and organisms in particular due to accumulation of these toxic compounds in their tissues. Histological and biochemical responses in organs of C. nigrodigitatus were used as effective biomarkers to evaluate the pollution status of Ologe and Badagry lagoons. Antioxidant enzymes are considered as sensitive biomarkers in environmental stress before hazardous effects occur in fish, and are important parameters for testing water for the presence of toxicants [23].
Increased oxidative stress has been suggested to enhance the antioxidant enzymes activities in fish, as a protective action towards oxidative stress. Superoxide dismutase (SOD) is one of the main antioxidant defense enzymes generated in response to oxidative stress. It converts the highly toxic superoxide anions into hydrogen peroxide. In the present study, SOD activity significantly increased (p < 0.05) in the gill of C. nigrodigitatus at Ologe and Badagry Lagoons. The pollutants in these Lagoons gives rise to higher production of superoxide anions which is countered by higher production of SOD enzyme in gills to neutralize the adverse effect. The antioxidant enzymes from this study have shown that the SOD activities in the organs work in a cooperative and synergistic manner to protect against oxidative stress and tissue-specific damage from stress-induced contaminants discharged into the lagoons. These enzyme systems have been proposed as biomarkers of reactive oxygen species (ROS) mediating contaminant exposure and as a potential tool in environmental risk assessment [23].
The enzyme SOD is known to provide cytoprotection against free radical induced damage by converting superoxide radicals (O2-) generated in peroxisomes and mitochondria to hydrogen peroxides. The hydrogen peroxide is then removed from the system by the enzyme CAT, which converts it to water and molecular oxygen (O2). The inhibition of the enzyme SOD leads to increased oxidative stress in tissues as a result of the damaging activities of the superoxide radicals (O2-) [24]. Furthermore, the inhibition of the enzyme SOD will expectedly result in a reduction in the activity of the enzyme CAT, due to a decrease in H2O2 generation from SOD activities [25]. This indeed proved to be the case in this study as there was a reduction in CAT activity from wet to dry season in the gill, liver and muscles of C. nigrodigitatus from Ologe Lagoon, as well as a reduction in CAT activity in gill and liver of the fish. Catalase (CAT) is a major primary antioxidant defense component that catalyses the decomposition of Hydrogen peroxides (H2O2) which is produced by the action of superoxide dismutase to H2O.
The GSH are non-enzymatic primary intracellular antioxidant agent represented by ROS scavengers. The depletion of GSH level at Ologe Lagoon, would probably be seem to enhance the risk of oxidative stress in liver and muscles of the C. nigrodigitatus. However, there was a significant increase in GSH of the fish observed across the seasons at Badagry lagoon. The depletion of GSH level is an indication that the Ologe lagoon is polluted, as compared Badagry lagoon which with slight pollution usually generated by domestic, dredging and fishing activities. This result agreed with those of Sharma, et al. [26] where they reported that, the glutathione metabolism contains crucial antioxidant molecules to defend the organisms against oxidants. GSH importantly contributes as a primary nucleophile in numerous protective detoxification reactions as also in the maintenance of cellular redox status [27].
MDA is used as marker of oxidation of membrane phospholipids through lipid peroxidation. An increase in MDA levels in organisms can be related to degradation of an environmental site by decreasing the water quality. An increase in level of lipid peroxides (measured as MDA), indicative of oxidative stress in the liver and gill of the fish exposed to admixtures of chemical constituent from the Ologe Lagoon, while in muscle the MDA decreased from the wet to dry seasons. The increases in as activities of MDA are due to an inhibitory effect on mitochondrial electron transport system leading to stimulation in the production of intracellular ROS [28]. However, this study observed a significant decrease of MDA in the gills, liver and muscle of C. nigrodigitatus at Badagry lagoon across the seasons, as indicative that there are less oxidative stress at Badagry lagoon. The environmental factors which play key roles toward the generation of oxidative radicals like ROS are minimally produced due to less human activities and industrial inputs which constitutes admixtures of chemical or xenobiotics were observed in Badagry lagoon, and therefore the measured MDA level were decreased in the organs of the fish species.
Histology is a useful technique towards the assessment of toxic substances in aquatic organisms from polluted ecosystems. Such a study also offers opportunity to locate the effect of pollutants in various organs and systems of animals. Variations in the epithelial surface of gills showed important physiological adaptations relating to the area available for increased gaseous exchange [29]. The lamellar reduction of the fish from Ologe lagoon must have been caused by respiratory stress as the sum of all the physiological responses by which an animal tries to maintain or re-establish a normal metabolism in the face of a physical or chemical force [29]. Cellular hyperplasia is an important feature in gills affected by pollution [30,31]. A number of pathological changes have been reported in fishes exposed to different heavy metals [32,33].
Liver are the main metabolic factory of the body serves several basic functions like metabolism, storage and secretion of bile. Liver accumulates more toxicants than other organs of body [29]. The morphology in the liver showed diffuse changes in hepatic tissue with disseminated moderate microvesicular vacuolation of hepatocytes, moderate vascular congestion and mild presence of eosinophilic fluid within the interstitial induced by admixture of chemicals from Ologe lagoon.
Kidney acts as a detoxifying organ by collecting and removing toxic materials along with nitrogenous waste products. Kumar, et al. [29] reported histological changes in kidney of Notopterus notopterus and Oreochromis mossambicus exhibiting degeneration and dissolution of epithelial cells of renal tubules, vacuolization, loss of nuclei, shrunken and ruptured glomerulus at some places, tubular necrosis, especially in the convoluted portion, and inflammation of glomerulus leading to impaired kidney functions like polyuria, polydipsia, and increased build-up of non-protein nitrogen. Renal tissue of C. nigrodigitatus from Ologe and Badagry lagoon showed mild haemorrhagic lesion, mild moderate ballooning degeneration of renal tubules and thickened blood vessel.
This study showed that there was an increased level of oxidative stress due to the presence of heavy metals alongside other toxicants induced by anthropogenic discharges comprising of admixtures of pollutants, and that an imbalance is generated between pro-oxidants and antioxidants as observed across seasons at Ologe Lagoon. However, the Badagry Lagoon poses less ecological risk with a decreased level of oxidative stress and a slight balance in pro-oxidant and antioxidant enzymes in the organs of Chrysichthys nigrodigitatus across seasons. The noticeable histological alterations in the organs of the C. nigrodigitatus are clear indications of the presences of xenobiotics or chemical compounds originated from industrial and other anthropogenic activities in Ologe lagoon. Therefore, the results of these studies also provide guidance to selection of tissues to be considered in the field of biomonitoring, designed to detect the bioavailability of aquatic pollutants and early-warning indicators of aquatic pollution in fish.