Recent reports have indicated a marked impairment of physical function in patients with acute cardiac disease. In addition, further deterioration in physical activity has been found during hospitalization especially in elderly patients, which may be associated with poor outcome after discharge. In this study, we repeatedly measured gait speed (GS) during hospitalization and evaluated the association of change in GS with mortality after discharge.
From January 2015 to October 2017, we enrolled 445 consecutive patients admitted to our hospital with congestive heart failure and undergoing exercise training during hospitalization. Physical examinations, including a 10 m walking test for measuring gait speed, were performed at the beginning of training (1st time point) and before discharge (2nd time point). Clinical parameters and clinical outcome after discharge during the follow-up period were compared between these groups.
Eighty-two participants (18%) showed a decline in GS even after training. In the linear regression analysis, age, poor activities of daily living (ADL) before admission, hand grip strength, controlling nutritional status (CONUT) score, tricuspid annular plane systolic excursion (TAPSE), change in hand grip strength and change in CONUT score were associated with the change in GS. Kaplan-Meier analysis showed the cumulative risk between groups in all-cause admission (log-rank test, p = 0.015) and all-cause death (log-rank test, p = 0.035).
Worsening gait speed during hospitalization was associated with poor outcome in patients with acute decompensated heart failure.
Gait speed, Heart failure, Elderly, Mortality
GS: Gait Speed; NT-proBNP: N-terminal pro-B-type Natriuretic Peptide; CONUT Score: Controlling
Nutritional Status Score; LV: Left Ventricular; LAVI: Left Atrial Volume Index; LVMI: LV Mass Index; TAPSE: Tricuspid Annular Plane Systolic Excursion; SD: Standard Deviation; ACE: Angiotensin-Converting Enzyme
The number of patients with heart failure increases rapidly in aging communities [1]. Despite the development of treatments for heart failure, the prognosis of heart failure is still poor, with high rate of hospitalization, readmission, and mortality [2-4]. In patients with acute heart failure, physical function is more impaired than in patients with stable heart failure [5]. In addition, further deterioration in physical activity has been found during hospitalization, especially in elderly patients, which may be associated with poor outcome after discharge. Repeated measurement of physical function during hospitalization may be helpful for risk stratification in patients with acute heart failure.
Gait speed (GS), a simple and useful assessment of physical function, is a predictor of cardiovascular events in the general elderly population [6] and in elderly patients with heart failure [7]. In this study, we repeatedly measured GS during hospitalization and evaluated the factors associated with the change in GS during hospitalization in elderly patients with acute heart failure who underwent exercise training. In addition, we followed these patients after discharge, and evaluated the association between the change in GS during hospitalization and mortality. We hypothesized that decline in GS during hospitalization is related to poor outcome after discharge.
From January 2015 to October 2017, we enrolled 445 consecutive patients who were admitted to our hospital with congestive heart failure. Patients with severe disabilities who could not perform the 10-m walking test were excluded. Heart failure was defined according to the modified Framingham criteria, as follows: Satisfaction of ≥ two major criteria (paroxysmal nocturnal dyspnea, orthopnea, rales, jugular venous distension, third heart sound, and radiological signs of pulmonary congestion and/or cardiomegaly), or one major criterion together with more than two minor criteria (effort dyspnea, peripheral edema, hepatomegaly, and pleural effusion). Diagnosis of heart failure was made by a cardiologist or an internist.
All patients underwent simple exercise training consisting of walking training and functional strength training of the lower extremities supervised by a physical therapist. Patients started training once they were able to walk without symptoms such as dyspnea. Blood pressure, pulse rate, and oxyopia saturation were measured before and after training. We performed the training in accordance with the standard cardiac rehabilitation program in patients with heart failure published by the Japanese Circulation Society in 2014 [8]. In this program, it is stated that all patients with acute heart failure may participate in cardiac rehabilitation programs when the patients' conditions are stabilized. Patients are advised to begin exercise training with stretching exercise of the limbs, low-intensity resistance training on the bed, standing position practice and tiptoeing on the bedside floor. After patient safety is confirmed, exercise training progresses to walking, cycle ergometer, light aerobics, and low-intensity resistance training. The study protocol was approved by the appropriate institutional review board of the hospital, and all participants provided written informed consent.
Physical ability was measured by a 10 m walking test to measure GS at the beginning of training (first time point) and at discharge (second time point). In the 10 m walking test, participants were asked to walk along a corridor at a comfortable speed. Participants were permitted to use walking aids such as canes and walkers. GS was calculated using the distance in meters and time in seconds. The maximum hand grip strength of the dominant hand was measured at both time points in kilograms using a handheld dynamometer. The best result of three attempts was recorded. A blood test was also performed at both time points to evaluate N-terminal pro-B-type natriuretic peptide (NT-proBNP) and nutrition status. NT-proBNP levels were measured using the commercially available Elecsys proBNP sandwich immunoassay with an Elecsys 2010 (Roche Diagnostics, Mannheim, Germany). Nutritional status was assessed by the controlling nutritional status score (CONUT) score [9] at both time points. The CONUT score was calculated using the serum albumin level (g/dl), total cholesterol level (mg/dl), and lymphocyte count (count/ml).
Echocardiography was performed in the left lateral decubitus position using a commercially available system during hospitalization. Left ventricular (LV) mass index and LV ejection fraction was calculated in accordance with the recommendations of the American Society of Echocardiography [10]. Peak velocities of E and A waves in mitral flow, the ratio of their peak velocities (E/A ratio), and deceleration time of the E wave were measured from the mitral flow velocity pattern. Tissue Doppler imaging of the mitral annulus was obtained from the apical four-chamber view as described previously [10]. Spectral pulsed-wave Doppler tissue interrogation of longitudinal mitral annular velocity was recorded throughout the cardiac cycle at the septal annulus in the apical four-chamber view. The peak of early diastolic (e' velocity) myocardial velocities was measured as an estimate of LV relaxation [11]. The ratio of E velocity and e' velocity (E/e' ratio) was calculated as an estimate of LV filling pressure [11]. Additional exploratory analyses, including changes in chamber dimensions and LVEF, were undertaken according to the recommendation of the American Society of Echocardiography [10]. Left atrial volume index (LAVI) was measured by the biplane area-length method, using measurements in apical 4- and 2- chamber views at end-systole and indexed by body surface area. The LV mass was calculated according to the Devereux formula and expressed as a ratio to the body surface area (LVMI) [10]. RV global systolic function was assessed as the tricuspid annular plane systolic excursion (TAPSE) [12].
Continuous variables were expressed as the mean ± standard deviation (SD) or the median with the interquartile range. Dichotomous variables were expressed as number and percentage. Patients were divided into two groups according to their change in GS between the two time points, with a cut off value of 0 m/sec: decreased GS group (n = 82) and increased GS group (n = 373). Differences in the continuous variables between the two groups were analyzed by Student's t-test and the Mann-Whitney U-test, as appropriate. Categorical data were compared by χ2 analysis and Fisher's exact test, as appropriate. In a subsequent analysis, the NT-proBNP data was log-transformed because they did not exhibit a normal distribution. Univariate and multivariate linear regression analyses were performed to evaluate the factors associated with the change in gait speed during hospitalization. In addition, univariate and multivariate logistic regression analyses were performed to evaluate the predictors of decline in GS during hospitalization. The multivariable analysis was conducted with adjustment using covariates with p-values less than 0.1 in the univariable analysis. Cumulative survival estimates were calculated using the Kaplan-Meier method and the data from the two groups were compared using the log-rank test. Statistical analyses were performed using SPSS V.24 statistical software (IBM, Armonk, New York, USA).
The mean GS increased from 0.58 ± 0.25 m/sec to 0.77 ± 0.33 m/sec (p < 0.001) between the two time points. At the first time point, 15 out of 455 patients (3%) had a gait speed of ≥ 1.0 m/sec, whereas 118 patients (26%) had a gait speed of ≥ 1.0 m/sec at the second time point. Among participants, 82 patients (18%) showed a decline in GS even after training (Figure 1).
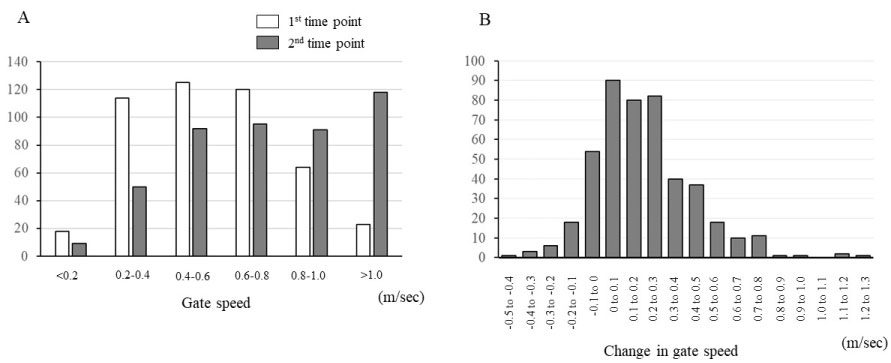 Figure 1: Distribution of gait speed at 1st and 2nd time points (A), and change in gait speed between the two time-points (B).
View Figure 1
Figure 1: Distribution of gait speed at 1st and 2nd time points (A), and change in gait speed between the two time-points (B).
View Figure 1
The patients' characteristics at baseline are shown in Table 1. The decreased GS group was older and had a higher prevalence of patients with low activities of daily living (ADL), defined as persons unable to go out by themselves. NYHA class was comparable between the two groups. In the blood test, hemoglobin was lower and NT-proBNP was higher in the decreased GS group. CONUT score was higher in the decreased GS group. In the physical assessment, hand grip strength and GS tended to be lower in the decreased GS group. In echocardiography, LVEF was comparable between the two groups, whereas TAPSE and e' was significantly lower in the decreased GS group. The changes in variables between the two time points are shown in Table 2. All these factors improved between the two time points in both groups, except for the hand grip strength in the decreased GS group. There was no difference in the use of medications for heart failure, such as angiotensin-converting enzyme (ACE) inhibitor, beta blockers and diuretics (data not shown).
Table 1: The patients' characteristics at baseline are shown. View Table 1
Table 2: The changes in variables between the two time points are shown. View Table 2
In the linear regression analysis, age, low ADL before admission, hand grip strength, CONUT score, TAPSE, change in hand grip strength and change in CONUT score were associated with the change in GS (Table 3). In multivariable logistic regression analysis, age, poor ADL before admission, e' and TAPSE were associated with decline in GS during hospitalization (Table 4).
Table 3: Change in GS: linear regression analysis. View Table 3
Table 4: Decline in GS: logistic regression analysis. View Table 4
During 3-year follow-up, 288 patients had at least one all-cause hospitalization. The all-cause readmission rate (76.1% vs. 61.1%, p = 0.0148) as well as all-cause death rate (15.9% vs. 8.8%, p = 0.044) was significantly higher in the decreased GS group than in the increased GS group. Kaplan-Meier analysis showed the cumulative risk between groups in all-cause admission (log-rank test, p = 0.015) and all-cause death (log-rank test, p = 0.035) (Figure 2).
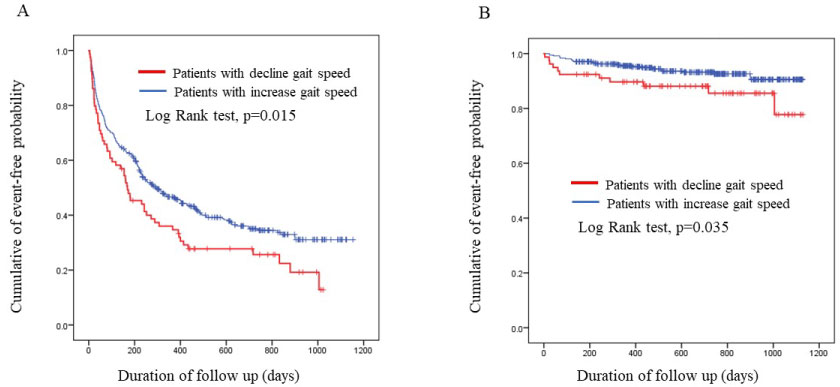 Figure 2: Kaplan-Meier curve showing freedom from all-cause readmission (A) and all-cause death (B) according to groups.
View Figure 2
Figure 2: Kaplan-Meier curve showing freedom from all-cause readmission (A) and all-cause death (B) according to groups.
View Figure 2
In this study, we repeatedly evaluated physical function using GS during hospitalization of elderly patients who were admitted with heart failure and underwent exercise training. Eighteen percent of patients showed a decline in GS, despite treatment for heart failure as well as exercise training. Nutritional status, hand grip strength and cardiac function were associated with the change in GS during hospitalization. Decline in GS was associated with higher incidence of clinical events, such as re-admission and death after discharge.
In our data, both muscle strength and nutritional status were related to the change in GS during hospitalization. In patients with heart failure, the prevalence of sarcopenia is higher than in patients without heart failure [13], and the complications of sarcopenia are associated with poor prognosis [14,15]. Sarcopenia progresses during hospitalization with heart failure, and heart failure causes an imbalance between anabolic and catabolic processes, which leads to a loss of muscle mass and function [16].
In our study, hand grip strength improved in patients with increases in GS, whereas there was no change in patients with decrease in GS despite the exercise training. In addition, change in hand grip strength was higher in patients with increase in GS. Although the precise evaluation of sarcopenia was difficult because we did not measure muscle mass, we thought that impaired muscle function may be involved in the decline in GS in these patients. Precise mechanism for the impairment of muscle function in patients with GS was not unclear; however, NT-proBNP was higher in patients with decline in GS than that in patients with increase in GS, which may indicate that severity of heart failure may be related to impairment in muscle function.
Malnutrition is common and is associated with greater mortality in patients with heart failure [6,17]. Malnutrition and muscle strength are related to each other. Disease-related malnutrition caused by lower appetite and malabsorption causes a loss of skeletal muscle mass and strength [18,19], which becomes highly prevalent in old age, and contributes to a greater risk of physical disability [20].
There are few data on the association of frailty and cardiac function. Leibowitz, et al. evaluated the association between cardiac function and ADL in community-dwelling older participants and reported that individuals with limitations in ADL had lower LVEF and higher LVMI [21]. In our data, LV diastolic function and RV function were associated with a decline in gait speed, whereas there was no association between LVEF, LVMI and change in GS, which was different from previous reports. The reason for the difference was not clear, however, we examined patients with acute heart failure and many patients had preserved LVEF, which may have been responsible for the difference. Previous reports demonstrated an association between LV diastolic function and frailty [22], however, there has been no data reporting an association between physical function and RV function. The specific mechanism for the relationship between these cardiac functions and decline in the physical function, both LV diastolic function [23] and RV systolic function, is related to exercise capacity [24,25], which may be involved in the association.
Poor ADL before admission was also associated with decline in GS in our patients. Low physical activity and leg strength were determinants of decline in mobility [26], Low mobility compounds muscle loss and physical deconditioning, and impairment in physical function due to low mobility is associated with adverse outcomes, even after controlling for illness severity [27].
In the present study, decline in GS during hospitalization was associated with future prognosis. Previous studies have demonstrated that GS was associated with mortality [6,7,28], however, the influence of the change in GS on future outcome has not been fully elucidated. Hardy, et al. reported that change in GS over one year was associated with future mortality in older subjects [29].
There are some possible mechanisms for the poor prognosis of patients with low physical function. Deterioration in physical function is associated with low skeletal muscle mass and function, and low nutrition, which may all contribute to poor prognosis in patients with low GS. Decreasing mobility may induce a vicious cycle of reduced physical activity and deconditioning that has a direct effect on health and survival [6]. Frailty is a risk for heart failure in the general older population [30] and in patients with stable heart failure [31]. Although the precise mechanism for the association between frailty and incidence of heart failure is still unclear, a previous study indicated that HF and frailty share a common pathophysiology that involves an inflammatory process [32]. In addition, patients with frailty have more cardiovascular risk factors [33] and, in fact, patients with slow gait speed have been found to have subclinical cardiovascular disease, such as increase in thickness of carotid intima-media and BNP [34,35].
Despite the development of treatments for heart failure, the outcome of heart failure is still poor due to a high re-admission rate [36]. Decline in physical function may be involved in the poor outcome in patients with heart failure. Preventing decline in physical function is a possible target for improvement in outcome after discharge in patients with heart failure.
It is inexpensive and simple to measure GS compared with other assessments. Repeated measurement of GS during hospitalization may be informative in identifying patients at high risk. Decline in GS may indicate a new health problem that requires further evaluation. In contrast, increasing gait speed predicts subsequent mobility, and physical function [37,38].
To prevent decline in GS during hospitalization, comprehensive interventions are needed, especially in patients with reduced cardiac function as well as poor ADL before admission. First, appropriate treatment for heart failure is important. Better implementation of pharmacotherapy is associated with better prognosis [39], and evidence-based medical therapies for heart failure are under used, especially in elderly persons [40]. ACE inhibitors prevent physical decline including muscle strength and walking speed in elderly persons [41].
Exercise training, including resistance training, is effective for improvement in muscle strength, which may contribute to preventing the decline in physical function [42]. Physical training is effective for improvement in muscle strength and function even in patients with frailty and dementia [43]. Maruya, et al. reported the natural decrease in physical function over a 6-month period in elderly patients with pre-sarcopenia or sarcopenia. This natural decline in muscle mass was associated with a decrease in maximum walking speed and muscle strength over the same 6-month period. In addition, a regular home exercise program (combination of walking and resistance lower limb exercise) is effective in preventing this decline in physical function [44].
In addition, we need to focus more attention on nutritional status to improve physical function in elderly patients. Nutritional interventions, such as the provision of high-protein oral nutritional supplements [45], might have a beneficial effect on the physical activity of elderly patients admitted with acute heart failure.
Several limitations should be considered when interpreting our results. We acknowledge that this was a single-center study. Thus, it is unclear if the findings can be extrapolated to other populations. There was a difference in patient characteristics, such as age, sex, level of ADL before admission, hand grip strength, and GS at baseline between the two groups. There is a possibility that these factors contributed to the association between the change in GS during hospitalization and the incidence of clinical events after discharge. In addition, the data on exercise-training progress were not available; thus, the effect of exercise training on the change in GS and mortality was unclear.
Worsening physical function during hospitalization is associated with poor outcome in patients with acute decompensated heart failure.
None.
The authors have no conflict of interest directly relevant to the content of this article.