Fucoxanthin, Fucoxanthinol, Fucoxanthin intake, Human serum, LC- MS/MS, MRM, Clinical trial
APCI: Atmospheric Pressure Chemical Ionization; Fx: Fucoxanthin; FxOH: Fucoxanthinol; HPLC: High-Performance Liquid chromatography; LC: Liquid Chromatography; LC-MS: Liquid Chromatography-Mass Spectrometry; LC-MS/MS: Liquid Chromatography-Tandem Mass Spectrometry; LLOQ: The Lower Limit of Quantification; LOD: The Limit of Detection; MCT: Medium-Chain Triglyceride; MRM: Multiple Reaction Monitoring; MS: Mass Spectrometry; m/z: Mass-to-Charge Ratio; Q1: The First Quadrupole; Q3: The Third Quadrupole.
Fucoxanthin is a marine carotenoid widely found in edible brown algae such as wakame (Undaria pinnatifida), kombu (Laminaria japonica) and akamoku (Sargassum horneri), and contributes more than 10% of the estimated total production of carotenoids in nature [1]. Fucoxanthin exhibits numerous health benefits especially anti-cancer [2-5] and anti-obesity [6,7] activities as well as benefiting various other health related problems [8-10]. According to Abidov, et al. in a report, which focuses on the anti-obesity activity of fucoxanthin, oral administration of a dietary supplement capsule containing fucoxanthin induces weight loss and increases resting energy expenditure in obese women [11]. However, only the activities of fucoxanthin were assayed and the kinetics of possible metabolites were unidentified in the report. To determine the physiological relevance of these activities, it is essential not only to study the digestion and absorption of orally administered fucoxanthin but also to identify its metabolites and the effective relationship between fucoxanthin and its possible activity.
Dietary fucoxanthin is incorporated into the blood as fucoxanthinol after deacetylation in the digestive tract [12] (Figure 1), and at least in the liver of mice, is then metabolized to amarouciaxanthin A [13]. Several detection systems have been described previously for analyzing fucoxanthinol in vivo, including HPLC [14,15], LC- MS [16,17] and LC-MS/MS [18]. Low bioavailability of fucoxanthinol compared to other carotenoids in humans has been demonstrated [14,15]. Asai, et al. reported that serum fucoxanthinol concentration after wakame intake (including 6.1 mg of fucoxanthin) was close to the lower limit of quantification by HPLC [14]. Therefore, to perform valid kinetics studies a more sensitive quantifying system for serum fucoxanthinol than exists at present is required. In addition to this approach, since the presence of highly concentrated proteins and other components in serum samples complicates analyses (e.g. causing background contamination by impurities), it is needed to remove them by improving preparation and analytical protocol to determine serum fucoxanthinol properly.
We aimed to develop a protocol for quantification of serum fucoxanthinol using liquid chromatography coupled with tandem mass spectrometry (MS/MS), which has a high sensitivity and a wide dynamic range. To apply the developed protocol to a large number of serum samples obtained from clinical trials, we also examined a washing step of LC for continuous analyses and evaluated the effects of pre-analytical factors, such as a freeze-thaw cycle on fucoxanthinol stability. We here report the kinetics in humans after a single dose of fucoxanthin to confirm the validity of applying the developed analysis to various clinical researches involving fucoxanthin intervention.
Fucoxanthinol, fucoxanthin and solvents for LC-MS/MS analysis were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), and other solvents were from Kanto Chemical (Tokyo, Japan). For clinical trials, fucoxanthin capsules were purchased from Kaneka Corporation (Osaka, Japan), prepared by Akamoku (Sargassum horneri) oil including 1% fucoxanthin and medium-chain triglyceride (MCT) oil including lecithin and vitamin E. Contents of the capsule were as follows; containing fucoxanthin 2.2 mg with 220 mg Akamoku oil and 20 mg MCT oil.
The LC system consisted of an Agilent 1100 series degasser, binary pump, auto- sampler, column oven (Agilent Technologies, Santa Clara, CA, USA) and Inertsil® - ODS column (4.6 mm i.d. × 150 mm, 3 μm, GL Sciences Inc., Tokyo, Japan). The HPLC mobile phases were methanol/acetonitrile (70/30, v/v) and elution was performed 0-5 min after sample injection (5 μL of standard fucoxanthinol and fucoxanthin or 50 μL of serum sample). The flow rate was set for 1 mL/min at 35°C.
The LC system was coupled with triple quadruple MS/MS 4000 QTRAP® LC/MS/MS system (AB SCIEX, Framingham, MA, USA), which was equipped with an atmospheric pressure chemical ionization (APCI) source, and operated in positive ion mode. The MS parameters optimized were as follows: declustering potential: 76 V; entrance potential: 10 V; dwell time: 400 msec; curtain gas (nitrogen): 10 psi; ion source gas 1 (nitrogen): 80 psi; turbo gas temperature: 500°C; interface heater: on; and nebulizer current: 5.0 psi. Nitrogen was used as the collision gas with a collision energy of 52 eV and a collision cell exit potential of 7 V. Analyst software 1.5 (AB SCIEX, Framingham, MA, USA) was used for the system control, data acquisition and data processing.
Calibration standards for fucoxanthinol were prepared at 1, 10, 100, 1000, and 10000 ng/mL by successive dilution with methanol/acetonitrile (70/30, v/v). Five microliter of each standard was subjected to LC-MS/MS as described above and the standard curve was constructed by plotting the peak area ratio of fucoxanthinol (y) versus the concentration (ng/mL) of fucoxanthinol (x).
Moreover, precision and accuracy of intra- and inter-day were assessed using calibration standards of 10, 100 and 1000 ng/mL (50, 500, 5000 pg on the column) fucoxanthinol and fucoxanthin. The precision and accuracy were determined by replicated analysis of 3 samples of each concentration of standard on 8 different days. Intra-day analysis was performed twice a day (i.e. morning and afternoon).
To quantify fucoxanthinol levels in serum samples, the serum epoxyxanthophyll fraction that included fucoxanthinol was extracted according to a method of Asai, et al. [14] with a slight modification. In brief, 1 mL serum, 0.2 mL saline, and 2 mL methanol including 5 ng fucoxanthin as an internal standard were added to glass tubes and vortexed. After adding 4 mL dichloromethane, the mixture was centrifuged at 416 g for 10 min at room temperature. The bottom layer was collected and this extraction procedure was repeated twice. The solvent of the collected fraction was removed to dry using a centrifugal concentrator (VC-96W, Taitec, Saitama, Japan) equipped with a vacuum pump and freeze trap. The residue was dissolved in n-hexane/diethyl ether (9/1, v/v), and applied to a Bond Elut ALN (100 mg, 1 mL) solid-phase extraction cartridge (Agilent Technologies, Santa Clara, CA, USA) pretreated with 1 mL n-hexane. After the cartridge was washed with the 1 mL n-hexane/diethyl ether (9/1, v/v), the epoxyxanthophylls were eluted with 1 mL diethyl ether/ethanol (4/1, v/v). The eluate was dried in vacuo, re-dissolved in methanol/acetonitrile (70/30, v/v).
After these procedures described by Asai, et al., we added to ultrafiltrate with Amicon® Ultra-0.5 mL 3K devices (Merck Millipore, Billerica, MA, USA) by twice centrifugation at 14,000 g for 10 min at room temperature, due to remove proteins carried over into collected epoxyxanthophyll fraction during extraction.
The collected sample was dried and resuspended in 250 μL of methanol/acetonitrile (70/30, v/v), and then the 50 μL sample was subjected to LC-MS/MS analysis. Triplicates of each serum sample were independently extracted and analyzed.
Fucoxanthin was used as the internal standard and spiked in serum to quantify the fucoxanthinol level because its structure and solubility are similar to fucoxanthinol. A previous report [15] and our preliminary experiment (Supplementary Figure 1) showed that fucoxanthin was not detected in serum after its intake. Samples were next analyzed in tuned MRM channels of both fucoxanthinol and fucoxanthin simultaneously and the amount of fucoxanthinol in serum was calculated from the area counts for fucoxanthin (it was assumed that the ionization efficiency of fucoxanthin and fucoxanthinol were similar in this quantitation system). Furthermore, the mean intra-day precision of serum sample analyses including the preparation procedure was calculated by the area counts of spiked fucoxanthin in the analyses among 32 days.
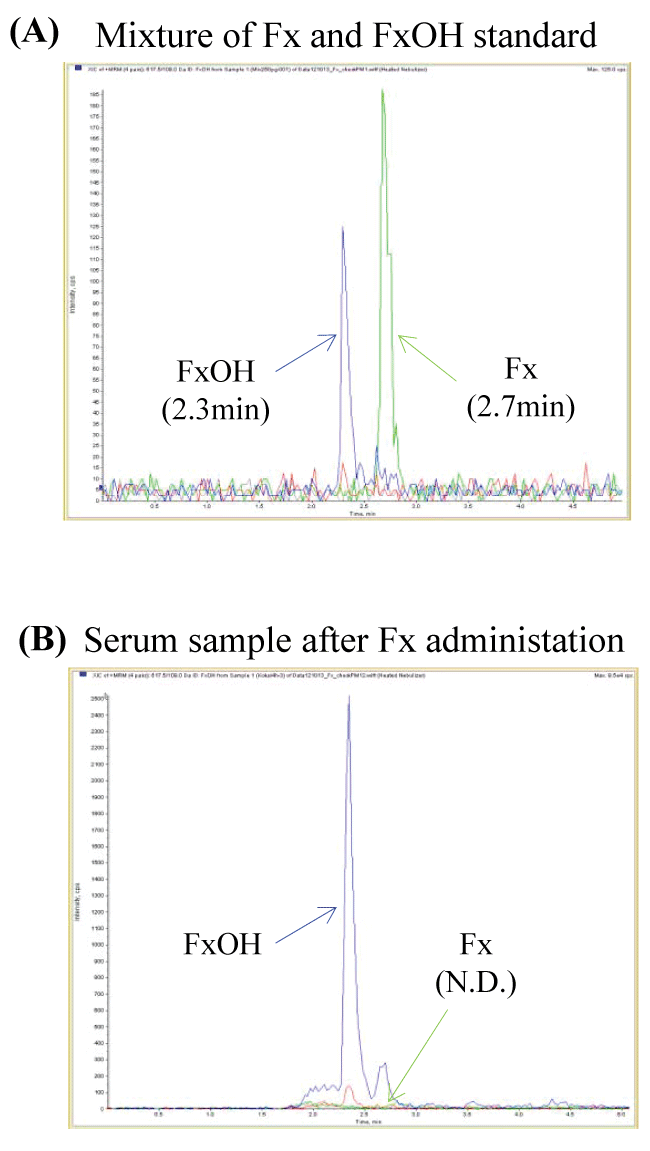 Supplementary Figure 1: Fucoxanthin was not detected in serum after its administration. After 4 hours of 22 mg of fucoxanthin intake, we analyzed fucoxanthin and fucoxanthinol in human serum by LC-MS/MS system. The peak of fucoxanthinol was detected at 2.3 min, that of fucoxanthin was not at 2.7 min. (A) Mixed solution of fucoxanthin and fucoxanthinol standard; (B) Serum sample after 4 hours of fucoxanthin administration.
View Figure 1
Supplementary Figure 1: Fucoxanthin was not detected in serum after its administration. After 4 hours of 22 mg of fucoxanthin intake, we analyzed fucoxanthin and fucoxanthinol in human serum by LC-MS/MS system. The peak of fucoxanthinol was detected at 2.3 min, that of fucoxanthin was not at 2.7 min. (A) Mixed solution of fucoxanthin and fucoxanthinol standard; (B) Serum sample after 4 hours of fucoxanthin administration.
View Figure 1
From these analyses, we determined the limit of detection (LOD) and the lower limit of quantification (LLOQ) in serum sample. The LOD and the LLOQ were defined as the concentrations with signal-to-noise ratios of 3 and 10, respectively [19].
Application of our assay to clinical research must identify variations due to changes in handling and processing of blood samples which may affect fucoxanthinol levels. To ensure optimal results for clinical fucoxanthinol levels, it is necessary to understand conditions which determine fucoxanthinol stability. Therefore, we systematically measured the impact of pre-analytical variables, particularly, freeze-thaw cycles (0 or 1 or 2) on fucoxanthinol.
Briefly, fucoxanthinol at concentrations of 250, 2500 and 25000 pg was spiked into 1 mL serum after blood collection and separation. Samples not freeze-thawed were immediately extracted as described above. In contrast, samples with 1 or 2 cycles of freeze-thaw were frozen at -80°C for 5 days or 12 days (5 days + 7 days), respectively. After freezing for each day, samples were thawed at room temperature and extracted. The 50 μL of extracted sample was subjected to LC-MS/MS analysis (i.e. 50, 500 and 5000 pg of theoretical fucoxanthinol values per inject). Triplicates of each concentration on freeze-thaw cycle were extracted and analyzed. The impact of freeze-thaw was analyzed by comparing it to fucoxanthinol on 0 cycle of freeze-thaw (expressed as 100%).
Subjects were 30~57 year-old healthy volunteers recruited from Sapporo Medical University (one woman and three men). The study protocols were approved by the ethical review committee of Sapporo Medical University (#24-2-91). Written informed consent was obtained from all subjects. Throughout the study, the subjects did not change their normal diets or physical activities.
In this experiment, subjects took a single dose of 22 mg fucoxanthin (in the form of 10 capsules). Blood was taken after 0, 4, 24 and 48 h of the dose and collected from a forearm vein and sat for 30 min in serum-separator tubes (with a coagulant). Sera were then centrifuged at 1,200 g for 10 min at room temperature and subsequently stored at -30°C until analysis. After one freeze-thaw cycles, epoxyxanthophyll fraction was extracted in triplicate by the method described above.
Results are expressed as mean ± SD or SE. Data comparisons were conducted using the Tukey HSD for multiple comparisons. The significance level was set at less than 0.05. Statistical analysis was performed using JMP10 by SAS.
To obtain greater sensitivity for quantifying serum fucoxanthinol, we employed multiple reaction monitoring (MRM) [20]. Because the most intense signals for fucoxanthinol and fucoxanthin were observed in the first quadrupole (Q1) and the third quadrupole (Q3) masses (Table 1) during continuous infusion of standard solution of each compound at 1 μg/mL, we selected the channel for fucoxanthinol (m/z 617.5, 109.0) and for fucoxanthin (m/z 659.4, 109.0) as quantitation ions (Table 1), respectively. We confirmed standard fucoxanthinol was detected in m/z 617.5 → 109.0 at RT 2.3 min (Figure 2A). The channel was linear in the concentration range tested of 10-10000 ng/mL (50-50000 pg on the column) with a correlation coefficient of 0.9989 (Figure 2B).
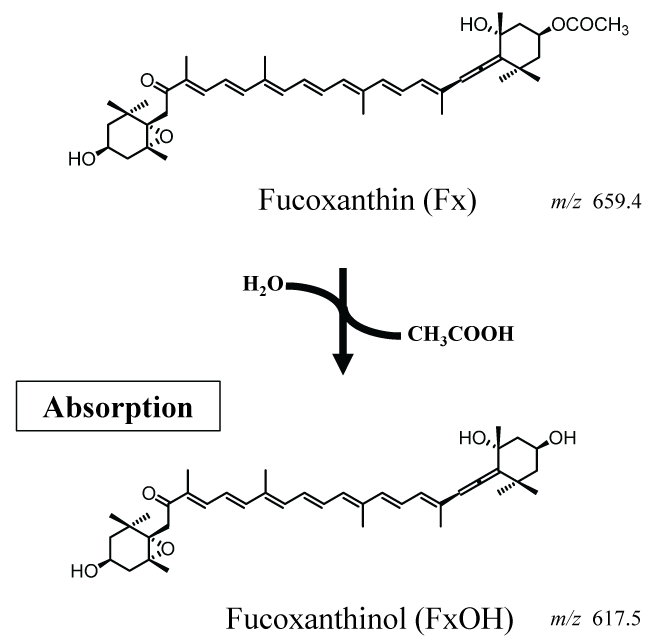 Figure 1: Biotransformation of fucoxanthin to fucoxanthinol in the digestive tract. Fucoxanthin is converted to fucoxanthinol, its deacetylated form, which is absorbed from the digestive tract into the circulation (Sugawara, et al. 2002)
View Figure 1
Figure 1: Biotransformation of fucoxanthin to fucoxanthinol in the digestive tract. Fucoxanthin is converted to fucoxanthinol, its deacetylated form, which is absorbed from the digestive tract into the circulation (Sugawara, et al. 2002)
View Figure 1
 Figure 2: Detection of standard fucoxanthinol by LC-MS/MS. (A) The chromatogram of fucoxanthinol (1000 ng/mL, i.e., 5000 pg on column) is detected by the tuned MRM channel. The channels of fucoxanthinol (as shown in Table 1) are expressed as follows: (blue line) a quantitation ion for fucoxanthinol, m/z, 617.5-109.0; (red line) a confirmation ion for fucoxanthinol, m/z, 617.5-67.0. Fucoxanthinol was detected at 2.3 min. The peak at 2.6 min might have been due to cis-isomer of fucoxanthinol [12]; (B) Linearity of standard curve of fucoxanthinol detected by the tuned MRM channel using a quantitation ion for fucoxanthinol.
View Figure 2
Figure 2: Detection of standard fucoxanthinol by LC-MS/MS. (A) The chromatogram of fucoxanthinol (1000 ng/mL, i.e., 5000 pg on column) is detected by the tuned MRM channel. The channels of fucoxanthinol (as shown in Table 1) are expressed as follows: (blue line) a quantitation ion for fucoxanthinol, m/z, 617.5-109.0; (red line) a confirmation ion for fucoxanthinol, m/z, 617.5-67.0. Fucoxanthinol was detected at 2.3 min. The peak at 2.6 min might have been due to cis-isomer of fucoxanthinol [12]; (B) Linearity of standard curve of fucoxanthinol detected by the tuned MRM channel using a quantitation ion for fucoxanthinol.
View Figure 2
Table 2 shows the results for precision and accuracy analyzed twice in one day or on 8 different days. The intra- and inter-day precisions in fucoxanthinol and fucoxanthin were shown in table 2. Generally, it is recommended that precision should be ˂ 20% and accuracy between 80-120% of the theoretical value. However, in the analysis of 10 ng/mL (50 pg on the column) of fucoxanthinol, both precision and accuracy were decreased in both intra- and inter-day sample analysis. On the other hand, for the concentrations of 100 and 1000 ng/mL (500 pg and 5000 pg on the column) of fucoxanthinol, the precision was ˂ 7% and the accuracy was 99.4-115.3%. Moreover, in all the concentrations of fucoxanthin, the precision was ˂ 17% and the accuracy was 95.5-101.8%.
Table 1: Candidates of MRM channels for fucoxanthinol and fucoxanthin. View Table 1
Table 2: Precision and accuracy of MRM channel of fucoxanthinol and fucoxanthin. View Table 2
To remove impurities from serum and shorten the total run time, we examined the effects of washing with dichloromethane and a reconditioning step from RT 5 min to 60 min. As shown in figure 3, peaks detected in MRM channels of fucoxanthinol (blue line; m/z 617.5, 109.0, red line; m/z 617.5, 67.0) were observed at least until 320 min under no washing step conditions. This was dramatically improved by washing with dichloromethane from 5 to 50 min. Figure 3B shows no peaks after 52 min. We thus successfully removed impurities and reduced total run time to less than 60 min. The final washing and reconditioning steps were as follows; solvent A (methanol/acetonitrile, 70/30, v/v) and solvent B (dichloromethane); 0-5 min with 0% B; 5-25 min with 0-100% B with a linear gradient of B; 25-50 min with 100% B and 50-60 min of 0% B. By adding the washing steps, we have developed a protocol (Figure 4) and achieved continuous analyses of serum fucoxanthinol in this study without any trouble such as a clogging of LC column. Although Zhang, et al. have reported the development of the system quantifying plasma fucoxanthinol in rats by LC-MS/MS, they did not mention washing conditions of LC column in continuous sample analyses [18]. Therefore, our examined washing protocol would provide the useful information for reasonable sample analyses.
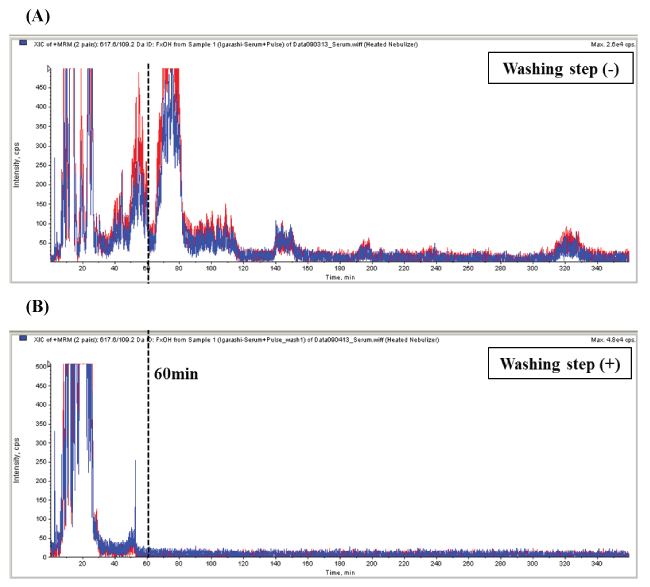 Figure 3: Examination of LC washing and reconditioning step for continuous analysis. Chromatograms were for analysis of serum sample (A) without and (B) with the washing step. Peaks detected in MRM channels of fucoxanthinol (blue line; m/z 617.5, 109.0, red line; m/z 617.5, 67.0) were observed at least until 320 min under no washing step condition (A). In contrast, washing with dichloromethane performed from 5 to 50 min (B) removed impurities after 60 min.
View Figure 3
Figure 3: Examination of LC washing and reconditioning step for continuous analysis. Chromatograms were for analysis of serum sample (A) without and (B) with the washing step. Peaks detected in MRM channels of fucoxanthinol (blue line; m/z 617.5, 109.0, red line; m/z 617.5, 67.0) were observed at least until 320 min under no washing step condition (A). In contrast, washing with dichloromethane performed from 5 to 50 min (B) removed impurities after 60 min.
View Figure 3
 Figure 4: A protocol for human serum fucoxanthinol quantitation.
View Figure 4
Figure 4: A protocol for human serum fucoxanthinol quantitation.
View Figure 4
Using the developed protocol, we analyzed clinical sample. The validation of fucoxanthinol and fucoxanthin in serum sample was shown in table 3. The mean CV of peak area of spiked fucoxanthinol and fucoxanthin were calculated as 9.2% and 19.2%, respectively. It indicated that this protocol kept good precision throughout serum sample preparations and analyses. In the recovery of fucoxanthinol and fucoxanthin, they were over 100% (Table 3). It may be due to the effect of serum contents. The LOD and LLOQ was fucoxanthinol and fucoxanthin from serum sample were 71.1 pg and 234.6 pg (in fucoxanthinol, respectively), and 36.2 pg and 119.5 pg (in fucoxanthin, respectively).
Table 3: The validation of fucoxanthinol and fucoxanthin in serum sample analyses. View Table 3
After a single dose of 22 mg fucoxanthin, its metabolite, fucoxanthinol, was measured in human sera at 0, 4, 24 and 48 h. No peak representing fucoxanthinol was detected in the MRM chromatogram at 0 h, while a rapid increase was observed at 4 h. Peaks declined at 24 to 48 h (Figure 5A). Fucoxanthinol was quantified using fucoxanthin as an internal control. Serum fucoxanthinol levels were markedly elevated at 4 h in all subjects and were gradually decreasing in 24 h to 48 h (Figure 5B). However, the levels did not return to the baseline of 0 h (0 h, 0.4 ± 0.2 nM; 4 h, 44.2 ± 14.9 nM; 24 h, 6.2 ± 0.9 nM; and 48 h, 1.6 ± 0.4 nM), suggesting that orally administered fucoxanthin is absorbed as fucoxanthinol within 4 h and is not metabolized completely even after 48 h.
 Figure 5: Changes in fucoxanthinol levels in human sera after a single 22 mg oral dose of fucoxanthin.
Figure 5: Changes in fucoxanthinol levels in human sera after a single 22 mg oral dose of fucoxanthin.
(A) Chromatograms of fucoxanthinol in serum at 0, 4, 24 and 48 h after a single dose of oral 22 mg fucoxanthin. In each instance, MRM channels of fucoxanthinol and fucoxanthin (as shown in table 1) are expressed as follows: (blue line) a quantitation ion for fucoxanthinol, m/z, 617.5-109.0; (red line) a confirmation ion for fucoxanthinol, m/z, 617.5-67.0; (green line) a quantitation ion for fucoxanthin, m/z, 659.4-109.0; (gray line) a confirmation ion for fucoxanthin, m/z, 659.4-581.3. Peaks of fucoxanthinol and fucoxanthin were found at 2.3 and 2.7 min, respectively; (B) Serum fucoxanthinol levels were determined at 0, 4, 24 and 48 h after a dose of 22 mg fucoxanthin in 4 subjects. Data are mean ± SE (n = 4).
View Figure 5
To prepare for handling freeze-thawed blood samples in clinical research, we examined the impact of freeze-thaw cycles by spiking standard fucoxanthinol. The 0 cycle of adjusted recovery of fucoxanthinol was expressed as 100%. As shown in figure 6, in serum samples of 5000 pg, the recovery of fucoxanthin was significantly decreased by freeze-thaw. Moreover, it seemed that 1 or 2 freeze-thaw cycles tended to decrease in each concentration, although a significant difference was not observed due to the wide SD of the 0 cycle sample. Previous reports indicate that retinol, alpha-tocopherol, trans- lycopene, and trans-beta-carotene in reconstituted lyophilized serum stored at -20°C were stable for at least 3 days with minimal (< 5) freeze/thaw cycles [21], however, fucoxanthinol in serum samples was less stable during freeze-thawing in our study.
 Figure 6: Impact of freeze-thaw cycle on stability of fucoxanthinol in human sera. After blood collection, fucoxanthinol (250, 2500 and 25000 pg) was spiked in 1 mL of serum and freeze-thawed 0 or 1 or 2 cycles. Recovery of fucoxanthinol in sample without freeze-thaw cycle was expressed as 100% and compared to freeze-thawed samples. Data are mean ± SD (n = 3).
Figure 6: Impact of freeze-thaw cycle on stability of fucoxanthinol in human sera. After blood collection, fucoxanthinol (250, 2500 and 25000 pg) was spiked in 1 mL of serum and freeze-thawed 0 or 1 or 2 cycles. Recovery of fucoxanthinol in sample without freeze-thaw cycle was expressed as 100% and compared to freeze-thawed samples. Data are mean ± SD (n = 3).
*Significantly different from 0 cycle of each concentration at P < 0.05 by Tukey's HSD.
View Figure 6
These results showed that repeated cycles of freeze-thaw should be avoided for fucoxanthinol quantification. It is impractical in clinical experiments to keep collected blood samples fresh until analysis. Unified freeze-thaw cycles would be important for analysis of a series of clinical samples.
We have developed a quantification protocol for fucoxanthinol in human sera as detected by LC-MS/MS and observed a change of fucoxanthinol levels after a single dose fucoxanthin. In this protocol, we have achieved continuous analyses of serum fucoxanthinol in this study without any trouble. These results may be applied to clinical investigations to elucidate the biological activity of fucoxanthin in humans.
This work was supported by a National Project for the Formation of Tohoku Marine Science Center (Innovation Strategy for the Industrial Seaweed Utilization Supporting Regional Seeds and Local Features of Sanriku Coast) from MEXT (Ministry of Education, Culture, Sports, Science, and Technology of Japan) and the Japan Society for the Promotion of Science Grants-in-Aid (JSPS KAKENHI Grant Number JP16K21251).
We would like to express our gratitude to Mr. Ryo Susukida for his technical support.
This study was conducted according to the guidelines of the Declaration of Helsinki, and our study protocol and all procedures involving human subjects were approved by the ethical review committee of Sapporo Medical University (#24-2-91).
The authors have no conflict of interests.
NM designed the research and performed experiments, analyzed and interpreted the data and wrote the manuscript. MH and MA designed the research, especially LC-MS/MS analysis, interpreted the data and revised the manuscript. KM formulated the study conception, interpreted the data and revised the manuscript. HS interpreted the data and wrote the manuscript. YK supervised the work, designed the research, interpreted data and wrote the manuscript. All authors read and approved the final manuscript.