Immune checkpoint inhibitors (ICIs) are a novel treatment modality that is quickly becoming the standard of care for the management of a multitude of neoplasms. While immunomodulation is a promising approach, it also carries a risk of immune-related adverse effects (irAEs). Nivolumab is an FDA-approved anti-programmed death-1 (anti-PD-1) checkpoint inhibitor. Nivolumab-induced, immune-related pancreatitis, and hyperbilirubinemia are rare but clinically significant examples of irAEs. Early recognition and prompt management of these conditions are critically important to ensure a better prognosis and prevention of subsequent complications.
Immune checkpoint inhibitors, Adverse event, Drug-induced pancreatitis, Drug-induced hyperbilirubinemia, Nivolumab
Cytotoxic T-cell lymphocyte-4 (CTLA-4) and programmed death-1 (PD-1)/ligand-1 (PD-L1) inhibitors have become a crucial part of the management of various malignancies. Due to their unique mechanism of action, these agents are called immune checkpoint inhibitors. It is estimated that 43.63% of all cancer patients in the US are eligible for treatment with ICIs, of which 12.46% of patients responded to therapy [1]. There is growing evidence that these agents confer a significant survival benefit in a wide spectrum of malignancies [2,3]. However, with increased use, an increase in the immune-mediated adverse effects of these agents referred to as irAEs, has also been seen. We report a unique case of acute pancreatitis and hyperbilirubinemia developing as an irAE of the use of Nivolumab immunotherapy.
A 62-year-old man presented to the emergency department with severe abdominal pain for the past two days. He also reported several episodes of vomiting and nausea. Two months back, the patient was diagnosed with squamous cell carcinoma of the lung metastatic to his vertebrae and was commenced on Nivolumab immunotherapy a month back. Laboratory results were significant for white blood cells 12,500/µL (3,300-8,600), amylase 232 U/L (42-130), lipase 279 U/L (16-58) aspartate transaminase (AST) 64 units/L (13-44),alanine transaminase (ALT) 72 units/L (8-66), total bilirubin 6.4 mg/dl (0.2-1.2), direct bilirubin 5 mg/dl (0.0-0.3 mg/dl) and C-reactive protein 2.3 mg/dl ( 0.00-0.16). Computed tomography (CT) scan of the abdomen demonstrated an enlarged, edematous pancreas with surrounding fluid and stranding (Figure 1). No gallstones or bile duct dilatation was noted. Due to high clinical suspicion, a hepatobiliary iminodiacetic acid (HIDA) scan was obtained, which came back normal. The patient had occasional alcohol intake. He was not taking any medications known to cause pancreatitis or hyperbilirubinemia except for Nivolumab. At this juncture, the patient was diagnosed with Nivolumab-induced pancreatitis and hyperbilirubinemia. Nivolumab therapy was discontinued. He was initially fluid resuscitated and was later started on high-dose steroid therapy with Prednisone at the 1 mg/kg/day dosing. The patient's symptoms significantly improved after 3 days of steroid treatment. He was prescribed a prolonged taper of oral Prednisone, tapered gradually over six weeks. On follow-up, the patient was asymptomatic and did not report any abdominal pain. Laboratory abnormalities had significantly improved with amylase 62 U/L, lipase 78 U/L, total bilirubin 1.8 mg/dL, and direct bilirubin 0.6 mg/dL on day 40 after treatment initiation.
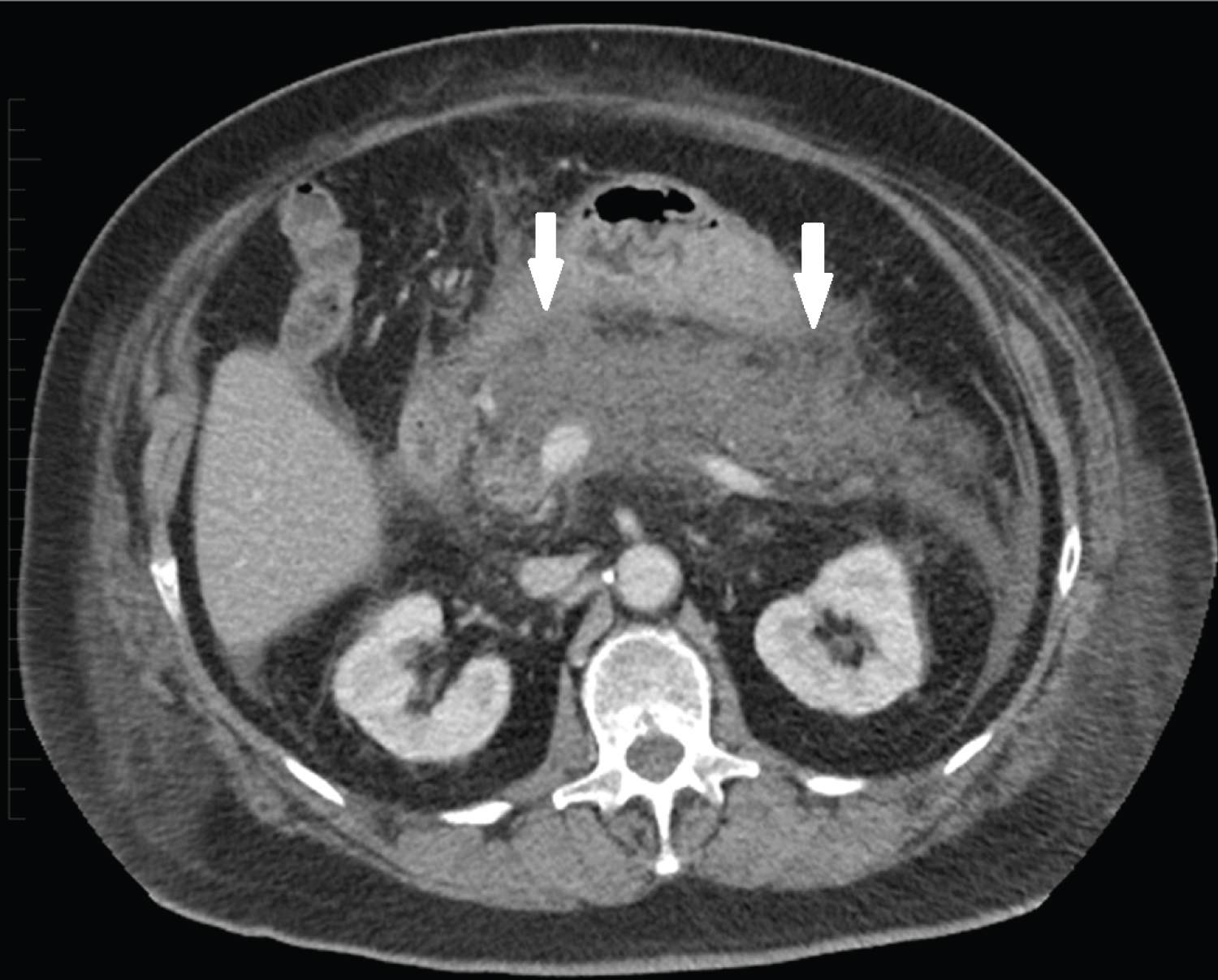 Figure 1: Axial CECT in a patient with abdominal pain demonstrates enlargement and edema of the pancreas with surrounding fluid and stranding, compatible with acute edematous pancreatitis. The predominant portion of the gland shows decreased intrinsic enhancement without definite necrosis.
View Figure 1
Figure 1: Axial CECT in a patient with abdominal pain demonstrates enlargement and edema of the pancreas with surrounding fluid and stranding, compatible with acute edematous pancreatitis. The predominant portion of the gland shows decreased intrinsic enhancement without definite necrosis.
View Figure 1
Suppression of T-cell mediated cytotoxicity is a well-known mechanism of oncogenesis, making immunomodulation with ICIs a promising approach for the management of a wide array of cancers. Anti-CTLA-4 and PD-1/PD-L1 blocking antibodies are the two commonly used classes of ICIs that are currently FDA-approved for the management of several malignant conditions. Nivolumab, a human immunoglobulin G4 monoclonal antibody (IgG4), selectively inhibits the PD-1/PDL-1 interaction by binding to the PD-1 receptor on T cells [4]. While this inhibition amasses a favorable anti-tumor immune response, it does not come without pitfalls. Firstly, the altered surface receptor interaction makes T-cells lose their ability to identify host cells. Secondly, while immune checkpoint inhibition has a stimulatory effect on effector T-cell proliferation, it also causes depletion of regulatory T-cells, removing a critical anti-inflammatory mechanism of the immune system [5]. Together these factors may cause infiltration of immune cells into normal tissues, significantly increasing the risk of irAEs. While skin and the gastrointestinal system are the most commonly affected, almost every organ in the body may be subject to these adverse events [6,7].
Immune-related pancreatic injury secondary to Nivolumab is a rare but important irAE with presentations ranging from an asymptomatic elevation in lipase levels to varying grades of clinically significant pancreatitis [7]. Immune-related pancreatitis may have a subacute, non-characteristic course and could be an underdiagnosed condition in patients with grade 3/4 elevation in amylase and lipase levels post Nivolumab therapy. A recent meta-analysis studied the incidence of drug-induced pancreatitis associated with ICIs. The study found an incidence of 2.7% for asymptomatic lipase elevation and 1.9% for grade 2 pancreatitis. A sub-group analysis to compare the incidence of drug-induced pancreatitis between the two classes of ICIs showed that therapy with anti-CTLA-4 agents was associated with a significantly higher incidence of pancreatitis (3.98%, 95% CI: 2.92-5.05) as compared to anti-PD-1 agents (0.94%, 95% CI: 0.48-1.40; p < 0.05) [8]. Characteristic symptoms and laboratory abnormalities in patients on Nivolumab should alert physicians to the possibility of this complication as early recognition and management can reduce associated morbidity and mortality. Nivolumab-induced pancreatitis largely remains a diagnosis of exclusion. A careful history and an abdominal ultrasound [9] can rule out common etiologies of pancreatitis, including alcohol use and gall stones. A temporal association of lab anomalies or symptoms with drug exposure may also help establish a causal relation. Previously reported cases have shown the onset of pancreatitis within 2-16 weeks of initiation of the drug [7].
Drug-induced hyperbilirubinemia has been commonly reported with isoniazid [10], amphotericin [11,12], allopurinol [12], and fluvastatin [13]. Nivolumab therapy has been associated with both hepatocellular and cholestatic patterns of hyperbilirubinemia, with the former being more common [14]. Cells of the biliary tract have an increased expression of human leukocyte antigen class 1 and 2, lymphocyte function-associated antigen 3, and intercellular adhesion molecule 1, making them susceptible to immune attacks in the setting of enhanced T-cell activity [4]. Similar to immune-related pancreatic injury, involvement of the biliary tract can also present on a spectrum ranging from asymptomatic hyperbilirubinemia, as seen in our case, to concomitant hepatotoxicity and cholangitis.
Most patients with irAEs respond to steroids [4,15] with previously reported cases of immune-related pancreatitis favorably responding to 1-2 mg/kg/day prednisolone [16]. Mycophenolate mofetil may be considered in steroid-resistant cases [4,15]. Infliximab, which has demonstrated efficacy in steroid-resistant irAE of colitis and pneumonitis, may also be a therapeutic option [16], except for cases with significant liver injury. A few cases have also demonstrated success with anti-thymocyte globulins, which act by depleting CD4 lymphocytes [17].
Nivolumab-induced immune-related pancreatic injury and hyperbilirubinemia are rare but significant irAEs that should be on the differential in a patient presenting with suggestive symptoms and laboratory abnormalities. Prompt treatment with immunosuppression usually shows a favorable response and may prevent complications. Further understanding of the predictive role of genetics and biomarkers hold significant potential for reducing irAEs.
The authors have no conflicts of interest to declare.
No financial and material support was taken.
No IRB approval needed.
Informed consent was obtained from the patient during an outpatient visit and was again obtained telephonically.
No content of the paper has been presented or published previously.
Literature review was done by all authors. Suryansh Bajaj and Unnati Bhatia wrote the first draft of the manuscript. The manuscript was critically revised by Darshan Gandhi and Divyansh Bajaj. Figure acquisition was done by Darshan Gandhi. All authors approved the final version to be published, and agree to be accountable for all aspects of the work.