Background: Bladder cancer is the most common malignancy of the urinary tract. Calcitriol [1,25(OH)2 vitamin D3] has anticancer effects mediated through binding to vitamin D receptor (VDR). The expression of VDR is present in many normal and cancer tissues. But there is little information about its expression in urinary bladder carcinoma. This study aimed to analyze VDR immunohistochemical expression in 74 Egyptian patients with urinary bladder carcinoma and to evaluate its association with different clinicopathological parameters.
Methods: Sections from formalin-fixed, paraffin-embedded tumor blocks were stained immunohistochemically using monoclonal anti-VDR antibody. VDR protein expression as well as its immunostaining patterns were recorded and scored separately in each case using semi-quantitative immunoreactive score.
Results: VDR was consistently expressed in the included histologically normal urothelium while tumor cells showed variable degrees of expression. Cytoplasmic/membranous VDR expression was common among the studied cases especially those with urothelial morphology (p = 0.076). While, the mean nuclear VDR was significantly (p = 0.007) higher in non-urothelial tumors. Nuclear VDR was significantly associated with muscle invasion (p = 0.000) and tumor stage (p = 0.001) in urothelial carcinoma. It was also statistically related to tumor grade, stage and muscle invasion in non-urothelial tumors (p = 0.002, 0.003 and 0.012, respectively).
Conclusion: There was a significant relation between nuclear VDR expression and prognostic markers suggesting its decrease as an indicator of a poorer prognosis. Vitamin D supplementation may represent a new treatment option for patients with bladder cancer.
VDR, Immunohistochemistry, Urinary bladder, Urothelial carcinoma, Non-urothelial carcinoma
AJCC: American Joint Committee on Cancer; LV: Lymphovascular; PN: Perineural; SCC: Squamous Cell Carcinoma; TNM: Tumor Node Metastasis; VDR: Vitamin D Receptor
Incidence of urinary bladder cancer is steadily rising worldwide, particularly in developed countries [1]. It ranks as the 10th most common cancer with estimated 573,278 new cases diagnosed and 212,536 deaths in 2020, according to GLOBOCAN data [2]. In Egypt, urinary bladder tumors represent 14.31% of total malignancies with higher incidence among men [3].
Urothelial carcinoma is certainly the most predominant histological type of bladder cancer [4]. But, other fewer common malignancies are encountered, including squamous cell carcinoma (2-5%), adenocarcinoma (0.5-2%) and small cell carcinoma (< 1%) [5]. These subtypes are generally associated with worse clinical outcomes compared to urothelial carcinoma [6].
Both tumor grade and stage are important factors in directing treatment decisions [7]. Given the serious complications induced by traditional treatment options for bladder cancer patients, it is critical to provide novel therapies with less side effects and acceptable outcomes [8,9].
Vitamin D is not only a hormone essential for calcium homeostasis [10] it also produces various biological effects through both genomic and non-genomic pathways [11]. The genomic pathway is mediated via binding of Calcitriol (active form of vitamin D) to vitamin D receptor (VDR) that belongs to the steroid-thyroid-retinoid receptor gene superfamily. VDR is mainly a nuclear receptor and has been identified in many neoplastic and non-neoplastic tissues. In non-genomic pathway, vitamin D activates a number of cytoplasmic signaling pathways that affect cell proliferation, differentiation and apoptosis and may act with the classical genomic pathway to trans-activate VDR [12-14].
Many in vitro and in vivo studies have shown the anticancer effect of Calcitriol and VDR in a wide variety of malignancies including bladder carcinoma [15], head and neck cancer [16], colon, breast and lung [13]. Thus, vitamin D supplementation, which is much less toxic and much more cost effective, deserves continued exploration for patients with bladder cancer [17].
In this viewpoint, we aimed to assess the immunohistochemical expression of VDR in different histologic subtypes of urinary bladder carcinoma and to evaluate the relation between VDR expression and the available clinicopathological characteristics.
Tumor samples from 100 patients with histologically proven primary urinary bladder carcinoma were received at the Pathology laboratory at specialized medical Center, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt between January 2019 and December 2020. All patients underwent a surgical procedure either radical cystectomy or transurethral resection of bladder tumor without receiving adjuvant chemo/radiotherapy before surgery. Out of them, 26 were excluded based on the following criteria: Inadequacy, poor processing, extensive necrosis, absence of muscularis propria in biopsies with invasive tumor, and pT2 in biopsy.
So, this study consisted of 74 specimens of bladder cancer, obtained by transurethral resection (n = 34 cases) and radical cystectomy (n = 40 cases). Formalin-fixed, paraffin-embedded tissue blocks were retrieved from the archives of the Pathology Department.
The available clinicopathological data including age, sex, grade, muscle invasion and pathologic tumor stage of cases were collected from the pathology request sheets enclosed with the specimens. Patients' data were completely anonymous, and their names were replaced by numbers. This study was approved by Beni-Suef University Ethical Committee (CFM-BSUREC/01122019) and was performed in accordance with the Declaration of Helsinki.
Hematoxylin and Eosin slides for each case were reviewed independently by two pathologists to confirm tumor histology and grade according to the WHO histological classification of tumors of the urinary tract [18], pathologic stage according to the Tumor Node Metastasis (TNM) system of the American Joint Committee on Cancer (AJCC), 8th edition [19] and lymph node metastasis in radical cystectomy cases. Details concerning the demographic and histopathologic characteristics of the studied cases are given in Table 1.
Table 1: Clinicalpathological characteristics and VDR protein expression in patients with urinary bladder carcinoma (n = 74). View Table 1
Immunostaining was performed on 4 µm thick sections of paraffin blocks using the streptavidin biotin peroxidase complex technique. Sections were incubated with ready to use mouse anti-VDR monoclonal antibody clone D6 (Medaysis, Catalog number #MC0304RTU7, San Francisco Bay Area, USA) for one hour at room temperature. In each staining session, a skin section was used as positive control for VDR antibody. Negative controls were done by replacing the primary antibody with phosphate buffer saline.
Evaluation of immunostained sections was performed by two independent authors blinded to all clinicopathological information.
Expression of VDR was assessed semi-quantitatively with regards to the intensity and the proportion of immunoreactive tumor cells. We recorded the percentage of tumor cells expressing VDR (regardless the pattern) in relation to the whole tissue area and graded as: 0, < 10%; 1, 11-30%; 2, 31-75%; 3, > 75%. Staining intensity was scored at four intensity levels: nil (0), weak/buff (1), moderate/yellow (2) or strong/intense brown (3). Then, the immunoreactivity score (IRS) was calculated by multiplying the values of these two categories and ranged between 0-9. Cases were considered negative (IRS 0-1), low expression (IRS 2-4) or high expression (IRS 6-9) [20].
We also assessed the intensity of staining and percentage of positive staining of VDR expression in the nucleus and cytoplasm separately in each tumor using the same IRS.
All slides were viewed using light microscopy (Olympus model BX53) while the included photographs were taken by Leica digital pathology slide scanner (APERIO LV1) at Pathology lab, Beni-Suef University hospital, Beni-Suef, Egypt.
The collected data were coded and statistically described in terms of mean ± standard deviation range, frequencies and percentages. Suitable tests were used for comparing categorical data and testing any significant correlation between VDR expression and other clinicopathological variables. P value of less than 0.05 was statistically significant. All analyses were performed using the statistical package for social sciences software for windows, SPSS version 18 (SPSS Inc, Chicago, IL, USA).
The mean ± SD age of the participants was 62.65 ± 10.9 years with a range of 35-77 years. The majority of cases were males and male to female ratio was 2.5:1. Urothelial carcinoma was the most common histologic type (n = 54, 73%) including 16 non-invasive low grade papillary carcinomas and 38 invasive high-grade ones. Out of which, 17 were conventional (pure) infiltrating tumors, 15 with squamous differentiation and 6 showed other divergent differentiation and variants. Among the urothelial cases, both pTa and pT1 were the most frequent tumor stage (29.6% each), followed by tumors invading the perivesical tissue (pT3) (22.2%). On the other hand, 20 patients had non-urothelial malignant tumors, 85% of which were muscle invasive (pT2 and pT3).
In this work, histologically normal urothelium was included in 29 cases. All showed high expression of VDR localized to the cell membrane and/or the cytoplasm (Figure 1). Meanwhile, all tumors expressed VDR in variable degree either in cell nucleus, cytoplasm and/or cell membrane with no recorded negative cases (Table 1). Chi-square test showed no significant association between VDR protein expression and any clinicopathological characteristics of urinary bladder carcinoma (p > 0.05).
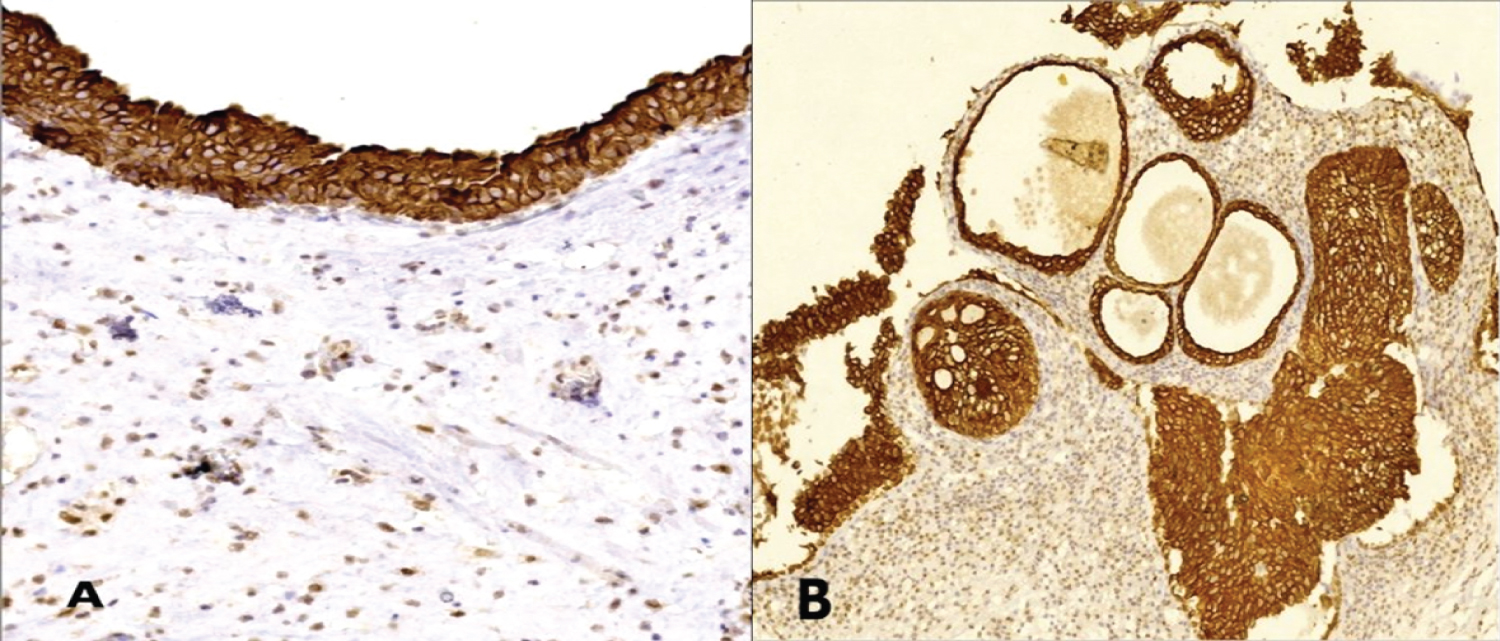 Figure 1: A) VDR expression in histologically normal urothelium and B) Urothelial proliferative changes shows ctoplasmic/membranous immunostaining (IHC, magnification A × 200, B × 100).
View Figure 1
Figure 1: A) VDR expression in histologically normal urothelium and B) Urothelial proliferative changes shows ctoplasmic/membranous immunostaining (IHC, magnification A × 200, B × 100).
View Figure 1
Overall, cytoplasmic/membranous and nuclear VDR expression were present in 68 (91.89%) and 45 (60.81%) cases, respectively. Strong immunoreactivity was commonly seen in cytoplasmic/membranous VDR expression (n = 35, 47.3%), as compared to 18 (24.3%) cases showed high expression of nuclear VDR (Table 2).
Table 2: Cytoplasmic and nuclear VDR expression in urinary carcinoma cases. View Table 2
Regarding the relation between tumor histology and VDR immunostaining patterns, the mean cytoplasmic/membranous VDR expression was higher in urothelial tumors than that in non-urothelial ones, however, this difference was statistically not significant (p = 0.076). The mean nuclear VDR expression was significantly (p = 0.007) higher in non-urothelial tumors (Table 3, Figure 2 and Figure 3).
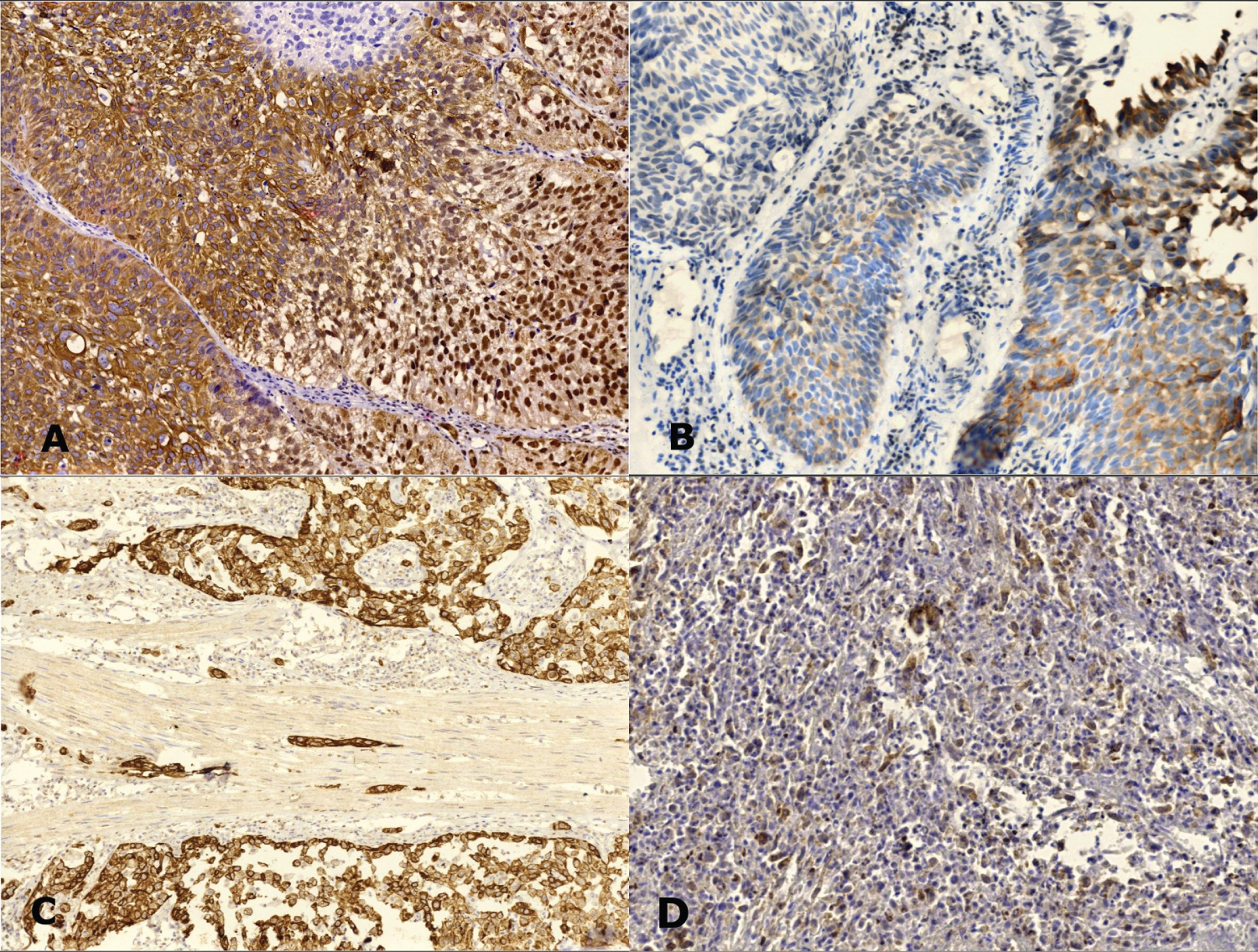 Figure 2: VDR expression in urothelial carcinoma cases: A) Non-invasive carcinoma shows strong cytoplasmic, membranous and nuclear staining; B) weak cytoplasmic staining. C) Muscle-invasive carcinoma shows strong cytoplasmic staining and D) weak staining (IHC, magnification A, B, D × 200, C × 100).
View Figure 2
Figure 2: VDR expression in urothelial carcinoma cases: A) Non-invasive carcinoma shows strong cytoplasmic, membranous and nuclear staining; B) weak cytoplasmic staining. C) Muscle-invasive carcinoma shows strong cytoplasmic staining and D) weak staining (IHC, magnification A, B, D × 200, C × 100).
View Figure 2
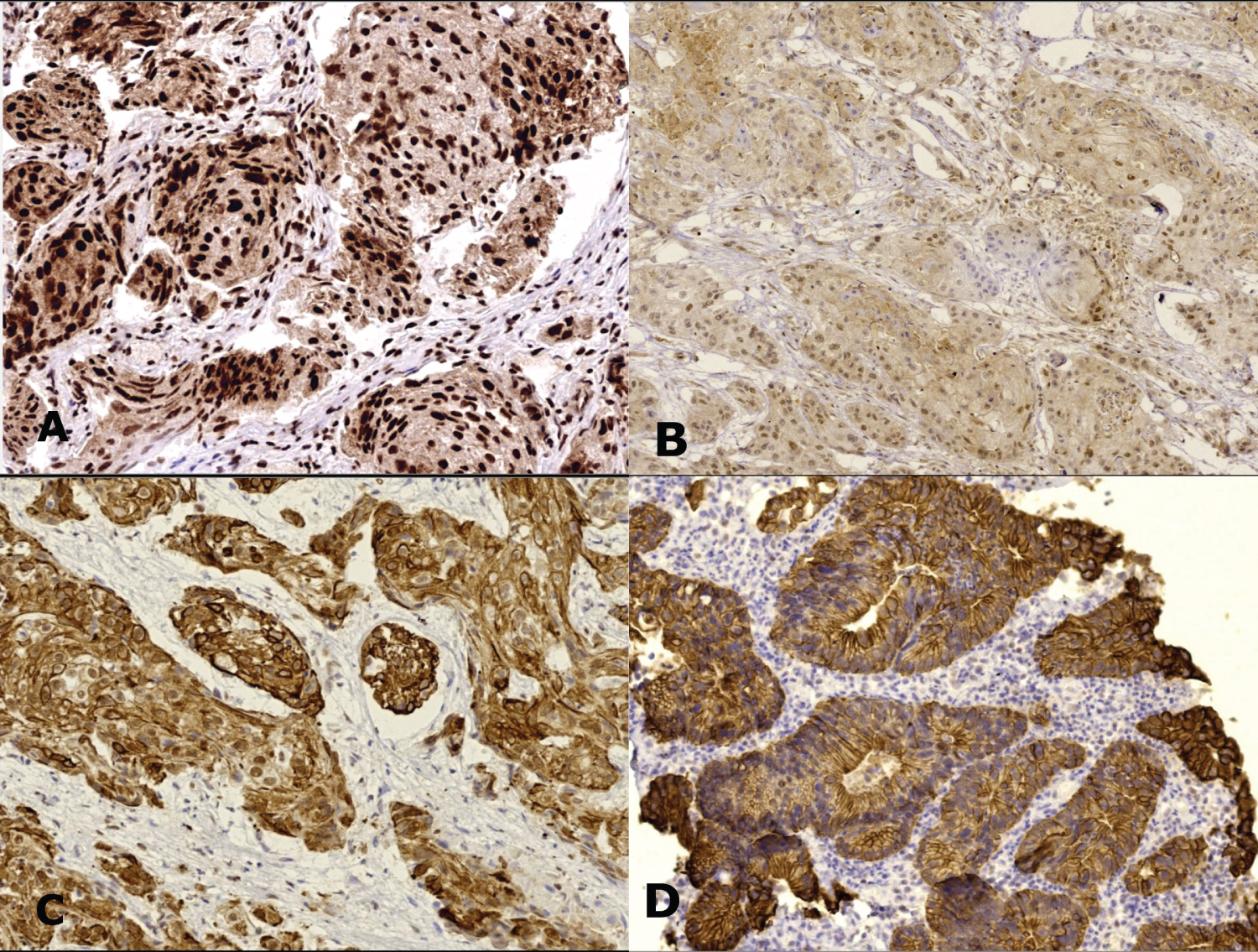 Figure 3: VDR expression in non-urothelial carcinoma cases A) Squamous cell carcinoma shows strong nuclear staining; B) Weak nuclear staining; C) High cytoplasmic expression. Primary bladder adenocarcinoma shows cytoplasmic/membranous expression (IHC, magnification A, C, D × 200, B × 100).
View Figure 3
Figure 3: VDR expression in non-urothelial carcinoma cases A) Squamous cell carcinoma shows strong nuclear staining; B) Weak nuclear staining; C) High cytoplasmic expression. Primary bladder adenocarcinoma shows cytoplasmic/membranous expression (IHC, magnification A, C, D × 200, B × 100).
View Figure 3
Table 3: The relationship of nuclear and cytoplasmic VDR with tumor. View Table 3
Among the urothelial carcinoma cases studied, there was a statistically significant association between muscle invasion, tumor extent and nuclear VDR expression (p = 0.000, p = 0.001, respectively). While, cytoplasmic VDR expression was not related to tumor grade, stage, muscle invasion or lymph node metastasis (Table 4).
Table 4: Major prognostic characteristics of urothelial carcinoma in relation to VDR expression patterns. View Table 4
The mean nuclear VDR expression in moderately differentiated and superficial non-urothelial cancers was higher compared to poorly differentiated and more advanced tumors. The differences were statistically significant. Cytoplasmic VDR in non-urothelial cases was also compared according to tumor grade, stage and nodal status but no relation was found (Table 5).
Table 5: The relation between the patterns of VDR expression and some pathologic features in non-urothelial carcinoma cases. View Table 5
Several immunohistochemical studies, so far, have been published to assess the relation between VDR expression and different types of cancers with variable and conflicting outcomes. Few studies focused on urothelial carcinoma of the urinary bladder [21]. To our best knowledge, we are the first to report VDR expression in non-urothelial tumors, as well.
Consistent with a study of 100 patients with lung adenocarcinoma by Kim [13], we observed no significant relations between VDR protein expression and all clinicopathological variables of urinary bladder carcinoma. While Anand [20], McCain [22] and Shi [23], reported a significant decrease in VDR immunoreactivity score across the AJCC anatomic stage/prognostic groups of patients with oral cancer, esophageal adenocarcinoma and colorectal carcinoma, respectively. All these studies did not evaluate the immunostaining patterns of VDR and their relation with histopathological parameters since several hypotheses found that the relationship between VDR expression and prognosis in cancer was mainly affected by the staining location.
This study revealed that VDR was consistently present in the cell membrane and the cytoplasm of normal urothelial cells. While cancer cells showed, in addition, nuclear immunostaining. Similar findings in normal colorectal cells were reported by Shi [23]. Absent nuclear staining in normal gastric mucosa and the underlying gastric and fundic glands was also noticed by Trowbridge [24]. However, Jóźwicki [25] found nuclear and/or cytoplasmic localization of VDR in all normal urothelial samples. Other cancer studies by Salehin [26] and Salomón [27] revealed nuclear localization in non-pathological vulvar tissues (n = 44/48) and non-malignant brain tissue (n = 3/3 positive samples), respectively, but in lower levels compared with the corresponding malignant tumors.
Overall, cytoplasmic/membranous VDR staining pattern was found to be more prevalent than the nuclear VDR among the studied cases. This observation agreed with those reported by Trowbridge (2012) [24], Zhou (2014) [28] and Shi [23]. Moreover, there was a trend towards increased cytoplasmic VDR expression in urothelial tumors (5.69 ± 2.99) versus non-urothelial ones of 2.85 ± 2.60, though p value did not yield significant. In contrast, Jóźwicki [25] found higher nuclear VDR levels (87.3%) in 71 patients with urothelial carcinoma. Different antibody clones used, different methods of staining, scoring and analyzing the VDR expression might explain the conflicting results.
Although VDR is mainly a nuclear receptor, it can be found in other subcellular structures as the cytoplasm and cell membrane. In the unliganded state, VDR remains in the cytoplasm [29]. Upon binding to Calcitriol, VDR translocation from the cytoplasm to the nucleus occurs with subsequent up or down regulation of hundreds of genes controlled by vitamin D [30]. Interestingly, VDR is also thought to mediate its molecular effect through a non-nuclear pathway in which VDR activates the MEK1/2/ERK1/2 pathway. The extracellular signal-regulated kinase (ERK) pathway is one of the major signaling cascades of the MAPK signaling pathway [31] that seems to be associated with urothelial tumorigenesis [32]. In colorectal cancer, cytoplasmic VDR expression was found to be associated with KRAS and PI3K mutations [33]. The RTK/RAS/PI3K pathway is altered in approximately 72% of cases with urothelial carcinoma [34].
For urothelial carcinoma cases, our work demonstrated that nuclear VDR expression was significantly reduced in muscle invasive and pT3 tumors compared with samples from early/superficial tumors. Meanwhile, a higher mean cytoplasmic VDR level was noted in non-invasive low-grade tumors than that in higher grade advanced disease. This difference was statistically not significant. Jóźwicki [25] reported the same finding but with a significant relation between cytoplasmic (not nuclear) VDR expression and tumor stage. Czogalla [31] displayed significant correlations between cytoplasmic VDR staining and some prognostic factors in patients with ovarian cancer.
We found high nuclear VDR expression among squamous cell carcinomas and adenocarcinomas of urinary bladder. Furthermore, nuclear VDR showed statistically significant differences in relation to tumor grade, muscle invasion and p (T) stage indicating its loss with increasing malignant progression. No studies of non-urothelial bladder cancer have discussed this point to compare with our results. However, comparable findings were reported by Srinivasan [21] where early and late stages of non-small cell lung carcinoma cases exhibited high nuclear VDR expression. Also, Salehin [26] observed higher nuclear VDR expression in well differentiated vulvar squamous cell carcinoma than the cytoplasmic VDR.
Such above results highlight the role of the classic nuclear pathway of VDR in regulation of genes that control inhibition of tumor development and suggest nuclear VDR as a potential prognostic marker for bladder cancer either urothelial or non-urothelial. This hypothesis is supported by a pilot study of human cancers that measured nuclear VDR concentration by an immunoradiometric assay and showed altered nuclear VDR number when a cell undergoes malignant transformation [35]. Given the chemopreventive effect of vitamin D analogs shown on breast cancer cells [36], gastric carcinogenesis [37] and other cancer types [38] in animal models, these may be suitable for treating patients with bladder carcinoma particularly those expressing nuclear VDR.
Limitations in this study include relative small sample size, and absence of follow up data of patients to evaluate the association of VDR expression with survival in bladder carcinoma. Also, we did not study vitamin D status of patients, but some authors found no significant correlation between serum 25(OH)D3 values and corresponding immunohistochemical VDR expression [16,39].
In conclusion, VDR is expressed in apparently normal urothelium and malignant tumors of urinary bladder; this may indicate an increased sensitivity to vitamin D-based therapeutic strategies. Nuclear localization of VDR was noted only in malignant cells. Nuclear VDR showed multiple significant relations with prognostic parameters in patients with urothelial and non-urothelial bladder cancer suggesting it as a potential biomarker for UBC.
Further larger studies correlated with patients' serum level of vitamin D are needed to prove our results. More research to investigate the role of VDR in premalignant bladder lesions are also recommended.
We express our sincere appreciation to departmental chair, all colleagues and technicians who helped us in this work.
The authors declare that there are no conflicts of interest.
Financial support is nil.
W.I, R.S. and B.M. performed study concept and design; R.S. and B.M. performed the literature searches and development of methodology; B.M., R.S., S.N. and W. I. provided acquisition, analysis, interpretation of data, and statistical analysis; R.S. and B.M. wrote the manuscript; S.N. and W.I. performed review and revision of the paper. All authors read and approved the final paper.