Introduction: Low back pain is a significant cause of disability worldwide. This study looks at the safety and efficacy of Autologous Adipose Derived Cellular Therapy in the form of Stromal Vascular Fraction (SVF) as a minimally invasive treatment option for low back pain.
Methods: This is a prospective, patient funded, IRB approved study looking at 549 patients who suffered from low back pain related to degenerative spinal conditions. The patients underwent SVF harvesting and deployment using a standard protocol. Data was collected using an online, automated database process.
Results: With a single SVF intervention significant pain improvement was documented out to 2 years from the time of intervention (79% of respondents reported significant pain improvement at 24 months). Adverse events were minimal and in line with or better than traditional minimally invasive therapies. No significant morbidity or mortality was identified.
Discussion: SVF appears to be a safe and effective therapy for low back pain caused by degenerative Lumbar Spine conditions.
Back pain is the leading cause of years lived with disability in the United States (US) [1]. Back pain is also a global issue with chronic spinal disorders being associated with lower quality of life around the world [2]. The cost burden associated with back pain is enormous. Health care and indirect costs are estimated at approximately $12 Billion per year in the United States alone [3]. Patients who suffer from back pain unresponsive to traditional conservative measures have a variety of options including epidural steroids, nerve blocks, neurotomy/ablation and surgical interventions (e.g. mirco/discectomy, laminectomy, fusion, etc.). These traditional therapeutic options for lumbar spine pain are associated with limited success and high risk [4]. Interestingly, none of these therapies mechanistically address the core underlying cause of pain - degenerative tissue processes. Such pathology can impact the disks, facet joints, ligaments and musculature of the back, all of which could be pain generators [5].
Regenerative therapies have been investigated as possible alternatives to traditional treatment modalities and have shown promise for patients suffering from spine related pain [6,7].
In this study we prospectively evaluate the safety and efficacy of an injectable, minimally invasive therapy for back pain. Autologous Adipose Derived Cellular Therapy, also known as Stromal Vascular Fraction (SVF), is isolated from a surgically obtained but minimally invasive lipoaspiration. SVF has been demonstrated to have anti-inflammatory and tissue regenerative capacity [8,9]. The potential to treat the underlying etiology of back pain disorders offers hope in a field currently dominated by symptom management and relatively high risk.
Patients with complaints of back pain who were either not responding to traditional therapy or who did not want to pursue traditional therapies were enrolled via affiliated clinics starting in 2012 through 2020. A total of 549 patients were enrolled to undergo interventional spinal injection(s) including: Transforaminal and interlaminar epidural injection, intradiscal injection, facet joint injection, spinal ligamentous injection(s) and/or intravenous administration of SVF.
Inclusion criteria: Patients over 18 years of age who had complaints of back pain either localized or radicular in nature that either was unresponsive to traditional measures/interventions, or if the patient elected to forgo such management.
Exclusion criteria: Active infectious process either local or systemic, pregnancy, coagulopathy, active or recent history of neoplastic disease, active enrollment in any other investigational study for back pain, active substance abuse.
This project is an IRB approved study by the International Cell Surgical Society Institutional Review Board. This trial is registered with ClinicalTrials.gov as NCT10953523. All patients were engaged in a shared decision-making process with informed consent signed prior to entering the study. Data was collected from study participants through a HIPAA compliant online database (TrackVia.com). Patients were followed for 2 years to assess pain scores, safety and complications considered to be associated with their investigational intervention. Cell Surgical Network (CSN) affiliated sub-investigators who contributed to this study are listed in the acknowledgement section.
The surgical isolation and processing of tissue has been previously described in detail elsewhere [10,11]. Briefly, all patients underwent a uniform mini-liposuction procedure consistent with the CSN IRB approved protocol. This included sub-dermal local anesthesia and syringe lipoaspiration (Medikan, South Korea). 50 mL of lipoaspirate was recovered from the harvest sites.
The lipoaspirate was condensed by centrifugation (2,800 rpm for 3 minutes), the infranatant cells and condensed fat were incubated with collagenase (Roche - 25 ml of 12.5 Wunsch units of GMP collagenase) at 38 °C for 30-40 minutes. Following this, the lipoaspirate was again centrifuged (200 RCF for 4 minutes - relative centrifugal force approximately 1,100 rpm). The supernatant was removed while maintaining the infranatant solution. Three successive washes using 50 ml of 5% dextrose lactated ringer solution (D5LR) and centrifugation were performed to effectively eliminate collagenase from the SVF. The resulting SVF was filtered through a 100-micron nylon filter producing 10 mL of concentrated SVF isolate and 50 mL of dilute SVF isolate.
SVF administration was performed using standard interventional techniques under sterile fluoroscopic and/or sterile ultrasound guidance for intradiscal injection, facet joint injection, epidural (transforaminal and interlaminar) injection and paraspinal ligamentous injections. Dilute SVF and any remaining concentrated SVF diluted into a total volume of 500 mL in D5LR and administered systemically as an IV infusion using an in-line 140 micron filtration drip set. In some cases, per physician discretion Platelet Rich Plasma (PRP) was added to the SVF being deployed intra-articularly.
Patient self-reported pain scores were obtained at baseline and followed at intervals of 3 months for a total of 2 years. Scores were described using the mean and standard error of the mean. The ANOVA method was used to analyze differences in the reported baseline and post-treatment pain scores.
For comparison, existing published data-sets documenting complication rates and efficacy of traditional minimally invasive standard of care therapeutic interventions (epidural steroids) and spinal surgical interventions were used [12,13].
A total of 549 patients were enrolled in this study. The gender breakdown was 303 males and 246 females. Average age was 66.35 years-old, the median age was 70-years-old (Table 1). Minimum and maximum and ages were 21-years-old and 92-years-old respectively. Patients were enrolled from October 2011 through May of 2021.
Table 1: Patients enrolled from October 2011 to May 2021. View Table 1
Deployment styles vary somewhat from provider to provider. 90.1% of deployments were performed via typical, fluoroscopically guided, standard of care injection practices along with IV deployment of the remaining SVF. 40.6% of the total deployments were fluoroscopically guided Lumbar intradiscal injections, 40.2% of the total deployments were fluoroscopically guided Lumbar facet injections, 1.7% of the total deployments were fluoroscopically guided transforaminal Lumbar epidural injections, 7.6% were non-image guided palpation/landmark injections into the paraspinal Lumbar musculature, the remaining 9.9% of patients received only IV deployment SVF. A total of 91.6% of all the patients received IV deployment of SVF with only 8.4% receiving only direct Lumbar injection of SVF without IV deployment.
Pain scores were reported on a 0-10 scale. 0 indicating no pain and 10 indicating maximal/excruciating pain. These scores were obtained at baseline (prior to the procedure), by the enrolling provider and thereafter obtained via email follow up with study participants. Pain scores were remeasured at 3-month intervals out to 24 month from the date of procedure (Table 2).
Table 2: Average participation and pain scores. View Table 2
Of the 549 patients, 87% reported baseline pain scores (N = 478). The mean baseline pain scores were reported for resting state 4.07, standing 5.56, walking 5.76 and while bending 5.66 (Figure 1). At one month post-procedurally 40% of patients reported average pain scores (N = 223). The 1-month pain scores were reported for resting state 2.34, standing 3.52, walking 3.67 and while bending 3.68. At 3 months to 9 months post-procedure, average participation and pain scores were variable and can be seen in Table 2. At one-year post-procedure, 17.5% of patients reported pain scores (N = 96). The mean reported pain scores were reported for resting state 2.54, standing 3.57, walking 3.57 and while bending 3.55. At 15 months to 21 months post-procedure, participation and average pain scores were variable and can be seen in Table 2. At 2 years (24 months) post-procedure, 11.8% of patients reported pain scores (N = 65). Two years (24 months) post-procedure, mean pain scores were reported for resting state 2.30, standing 3.45, walking 3.24 and while bending 3.06.
 Figure 1: Back-Lumbar improvement in condition reported by percentage.
View Figure 1
Figure 1: Back-Lumbar improvement in condition reported by percentage.
View Figure 1
The Data from Table 1 and Table 2 are represented graphically as well as the Standard Error of the Mean in Figure 2.
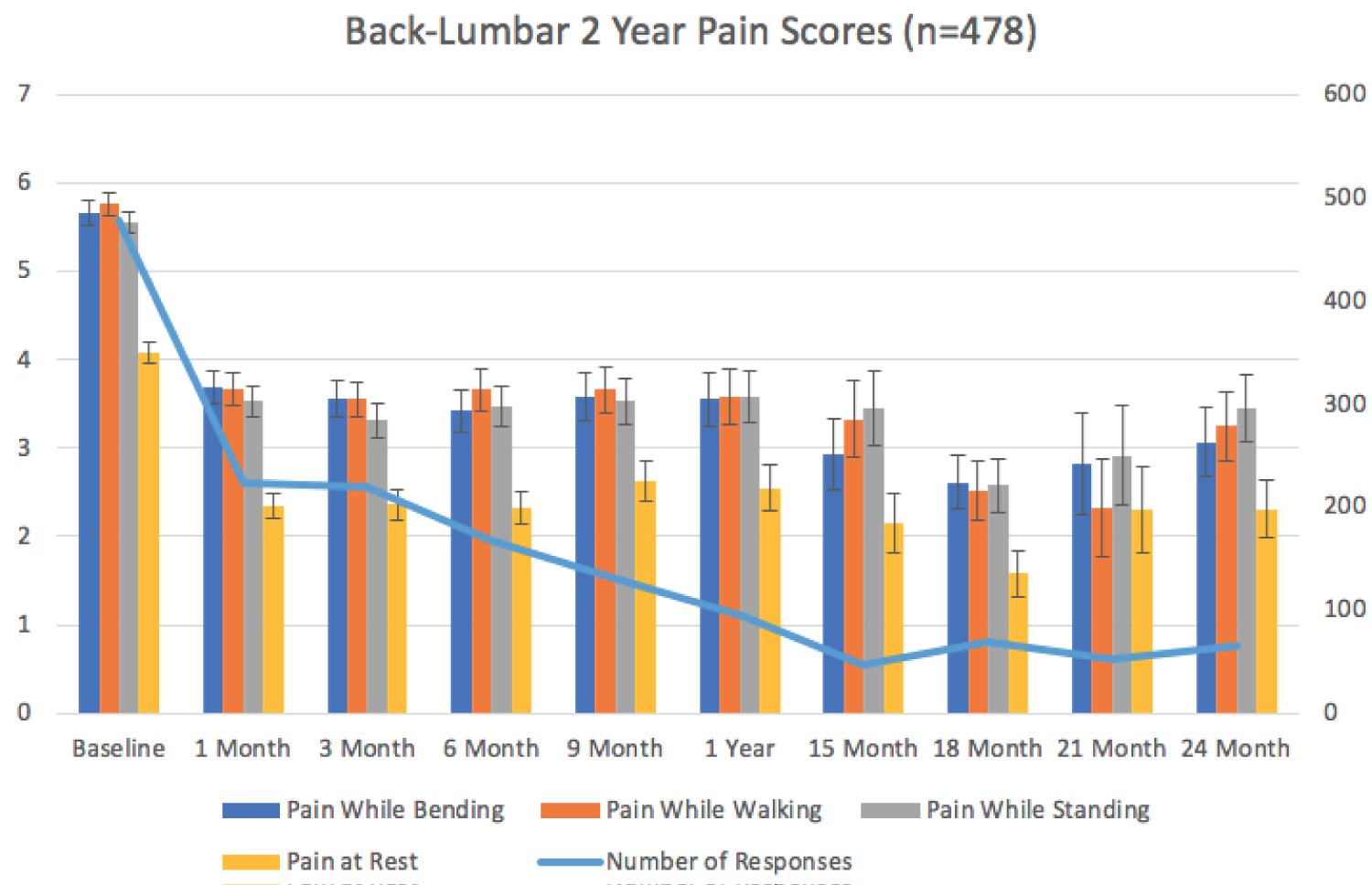 Figure 2: Back-Lumbar 2 year pain scores (n = 478).
View Figure 2
Figure 2: Back-Lumbar 2 year pain scores (n = 478).
View Figure 2
The difference between the reported baseline pain scores compared with all other time point pain score averages are statistically significantly different with P values of less than 0.05 (ANOVA). At 1 year (12 months) P = 0.00511936. At 2 years (24 months) P = 0.04198684.
Patients were asked if they felt that they had experienced improvement as a result of their intervention. The results of the reported responses are represented in Table 3. At all follow up time points, the majority of the patients reported improvement in their pain. One month post-procedure, 73% reported improvement (N = 233), 3 months post-procedure, 78% reported improvement (N = 220), 6 months post-procedure, 81% reported improvement (N = 168), 9 months post-procedure, 81% reported improvement (N = 130), 12 months post-procedure, 66% reported improvement (N = 96), 15 months post-procedure, 81% reported improvement (N = 46), 18 months post-procedure, 87% reported improvement (N = 69), 21 months post-procedure, 95% reported improvement (N = 52), 24 months post-procedure, 79% reported improvement (N = 65). These data are represented graphically in Figure 1.
Table 3: Results of the reported responses. View Table 3
Of the 549 patients who underwent treatment, a total of 22 complaints were reported in the tracking system which patients felt were a result of the intervention. These complaints were reviewed by the investigators and of these, a total of 10 were judged to be true adverse events while 12 were judged as reasonably expected outcomes of the procedure. The 12 reasonably expected outcomes comprised 7 reports of pain during the surgery/harvesting/procedure and 5 reports of increased inflammation at the deployment site. All 5 episodes of increased inflammation resolved spontaneously within 72 hours of deployment. The 10 complaints that were considered by the investigators to be true adverse events involved 5 reports of “unspecified” adverse events where no further clarification or explanation was able to be obtained from the patients and 5 reports of surgical harvest site wound infection. All 5 surgical site infections were managed successfully with oral antibiotics. There were no serious adverse events reported such as need for hospitalization, need for follow up surgery, neurologic deficit, discitis, thromboembolic events, epidural hematoma or abscess, mortality or other serious complications (Table 3).
Neoplastic events were tracked as part of the follow up. A total of 8 novel neoplastic events were reported in the database during the 2 year follow up period. There were 3 reports of squamous Cell Carcinoma, 2 reports of Prostate Cancer, 2 reports of Breast Cancer and 1 report of Lung Cancer (Table 4). None of these neoplastic events were harvest or deployment site events and none were metastatic in nature at the time of reporting.
Table 4: Adverse events report. View Table 4
In lieu of an internal control, external comparators of existing published data-sets were used. As a minimally invasive spine intervention lumbar epidural steroids were used as a comparison using the Kennedy, et. al. data published in The Spine Journal in 2017 [12]. For long term neoplasm comparison, NIH Surveillance Epidemiology and End Results (SEER) Program data was used [14].
The Kennedy, et al. data-set looked at patients with unilateral, lumbar, radicular pain due to disk herniation and their response to lumbar transforaminal epidural steroid injections. In this study 78 patients were enrolled, average age was 35.8 years, these patients were followed for 5 years. At the 5 year follow up, there was 50% participation. This data-set demonstrated that 76.8% of the patients developed recurrent pain (Figure 3), 29% required repeat steroid injection and 48.7% ultimately underwent surgery for their pain (Figure 4) [12].
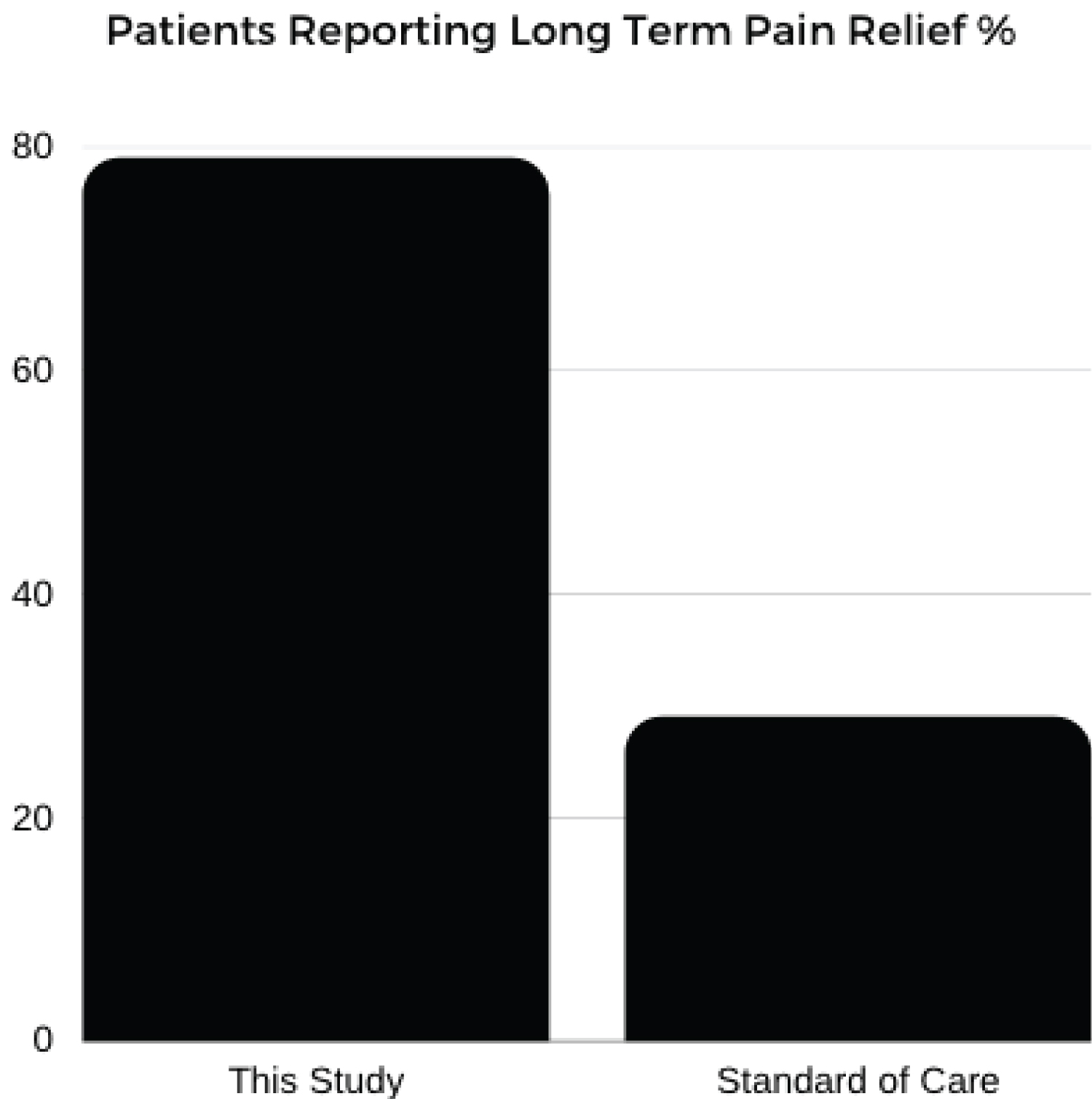 Figure 3: Patients reporting long term pain relief %.
View Figure 3
Figure 3: Patients reporting long term pain relief %.
View Figure 3
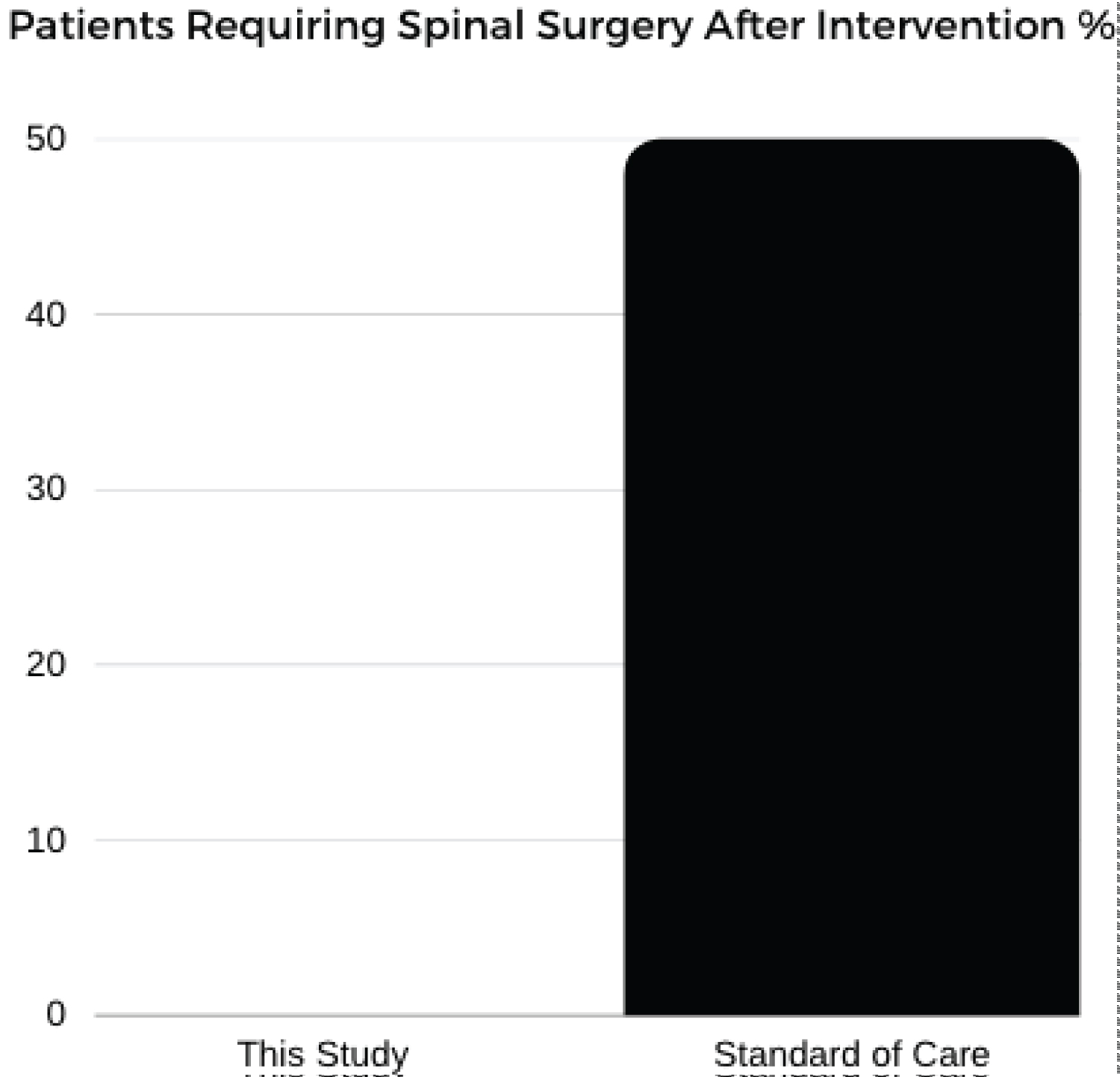 Figure 4: Patients requiring spinal surgery after intervention %.
View Figure 4
Figure 4: Patients requiring spinal surgery after intervention %.
View Figure 4
Our data demonstrated 79% of patients reporting pain improvement at 2 years and no patients reported need for follow up spinal surgery (Figure 3 and Figure 4).
SEER data demonstrates that in the US, in the 6th decade of life, annual risk of receiving a diagnosis of cancer is 1.3% per year. Over 2 years this equals 2.6% risk of a new cancer/neoplasm diagnosis. Also, the most common cancer diagnoses in the US in descending order are: Breast, Prostate, Lung, Colon, GYN, and then Skin [14].
Based on the above statistics, a comparison of US cancer data to this study’s findings demonstrates that expected risk of age matched, new neoplastic events should be 2.6%. In our study population a total of 1.46% of participants reported a new cancer diagnosis (Figure 5).
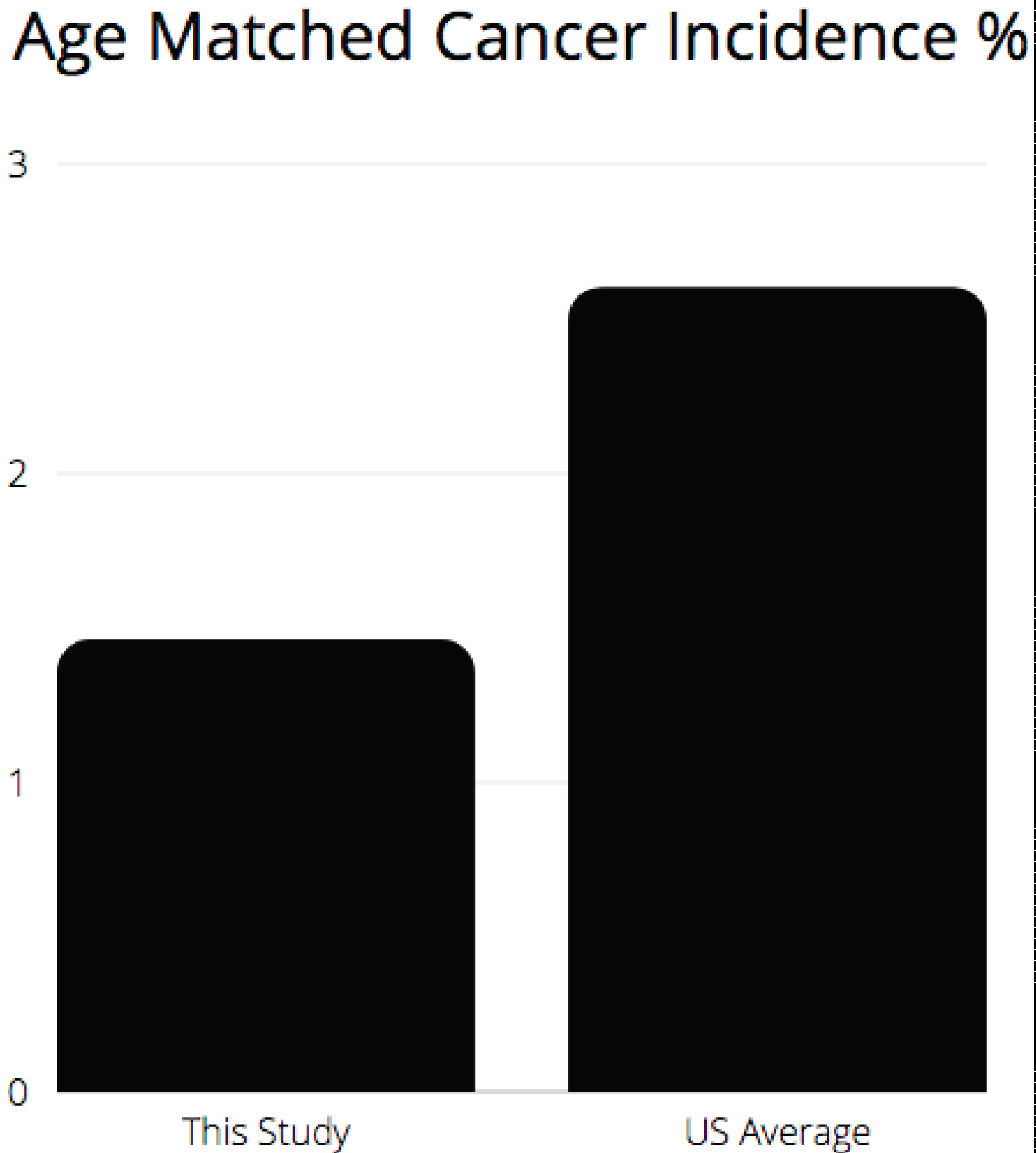 Figure 5: Age matched cancer incidence %.
View Figure 5
Figure 5: Age matched cancer incidence %.
View Figure 5
Table 5: Report of neoplastic events. View Table 5
This study demonstrates that long term, two-year, significant pain relief from low back is achievable with a single intervention of autologous adipose derived cellular therapy. When compared with traditional minimally invasive interventions, autologous adipose derived cellular therapy appears to provide a much longer period of pain relief [12] which could potentially reduce the number of patients who require surgical intervention for low back pain related disorders.
The above cited comparative data-set [12] and other data-sets [15] demonstrate that steroids are not effective for long term pain control in low back pain. Although pain is improved in the short term with steroid injections, long term follow up shows that 76.8% of patients develop recurrence of pain, 29% require repeat steroid injections, and ultimately 48.7% of patients go on to require surgery [12]. Based on this comparative data, autologous adipose derived cellular therapy, when compared with steroid injection, appears to provide a significantly longer period of pain relief. One could postulate that cellular therapy could serve as an intermediary treatment step between steroid injections and surgical intervention. If that were the case, cellular therapy could reduce the number of patients needing surgical intervention for low back pain related disorders. We feel this is important to consider, particularly relating to another common refrain about cellular therapy which is cost. The cost of cellular therapy is higher than steroid injections, but it is significantly less than the cost of surgical intervention [16]. Autologous adipose derived cellular therapy also appears to be much safer than surgical intervention, particularly when considering that surgical intervention carries a mortality risk in addition to morbidity risks [17].
This study demonstrates that intervention in the lumbar spine with autologous adipose derived cellular therapy is safe with minimal short term complication rates. The most common complications identified in this study - pain, inflammation and surgical site infection are extremely rare. The cases of surgical site infections were universally managed with oral, outpatient antibiotics, without need for further intervention. It is important to note that the SSI rate in this study of 0.91% is actually lower than nationally accepted norms for SSIs, which are generally considered acceptable at a rate of 1-3% [18-20]. Furthermore, This study found no incidence of thromboembolic disease. Venous thromboembolism (VTE) is a well-known complication associated with spinal surgery where the rate VTE is documented at 13% of all people undergoing spine surgery with Pulmonary Embolism at 8% and Deep Venous Thrombosis at 6% [21].
This study also directly addresses long term cancer risk associated with cellular therapy. It is important to note that other long term studies have previously documented that cellular therapy does not appear to put people at risk for neoplastic disease [22]. This study demonstrates that the treatment group at two years from the time of intervention was not at increased risk for neoplastic disease when compared with the age matched general population. This study’s treatment group actually developed neoplastic disease at lower rates than the aged match population. We do not claim that cellular therapy is protective, however, this would be an interesting area of study for future investigation.
Limitations of this study include the attrition in the study group respondents, self-reporting of results, operator heterogeneity in the deployment methods and a lack of internal control or blinding. We have attempted to address the issue of lack of internal control by using an existing published data-set on a standard of care alternative to cellular therapy. Going forward, we are addressing the issue of participation attrition by altering the data collection methods in an attempt to limit attrition. It is important to note that the standard of care data-set also suffers from long term attrition. Not only did that study start with a much smaller initial N of 78 but that data-set suffers from a 50% attrition rate.
In this study we present the largest ever, long term study looking at the treatment of low back pain using a biologic therapy in spinal disorders. This data demonstrates that autologous adipose derived cellular therapy appears to be a safe and effective minimally invasive therapy for long term pain relief for degenerative disorders in the lumbar spine. These results are promising though further study is needed. Ideally future studies should be performed to attempt to replicate these results as well as to further elucidate issues such as ideal candidates, timing of therapy and frequency of treatment.