Background: The aim of the study was to investigate the therapeutic efficacy of Coartem® in the treatment of uncomplicated P. falciparum malaria in Woreta Town, South Gonder Zone, Ethiopia.
Methods: 2240 febrile patients attending the health center were screened and capillary blood was obtained by finger prick. Giemsa stained thick and thin blood smears were prepared and used for parasite density and species identification. Of the 2240 patients tested, 88 with confirmed falciparum malaria were enrolled and treated with Coartem®. Haemoglobin concentration of the study participants was measured on day 0, 14 and 28. Of the 88 patients enrolled, five were lost to follow and five were excluded from treatment response analysis due to protocol violation. As a result, 78 patients were evaluated for treatment outcomes.
Results: The adequate clinical and parasitological response was 100% at day 28. Fever clearance was fast with only 1.7% being febrile at day three. Parasite clearance was rapid with almost all patients (98.9%) being free of parasitmia on day 2. A significant increase in haemoglobin level was observed on day 28. Coartem is safe and well tolerated for the treatment of uncomplicated P. falciparum malaria in the study area.
Conclusion: Thus, these findings further support the use of Coartem as an effective and safe treatment of uncomplicated P. falciparum malaria in Woreta.
Coartem®, Cure rate, Fever clearance, Parasite clearance, P. falciparum
Malaria remains one of the most widespread parasitic diseases in tropical and subtropical regions of the world. The estimated number of cases of malaria in 2011 was 216 million. There were an estimated 655,000 deaths each year in the world, of which 91% were in Africa [1,2].
In Ethiopia, 75% of the area is malarious; making the disease major public health problem in the country. One of the challenges of controlling malaria is the evolution of resistant strains of Plasmodium against antimalarial drugs [3]. Coartem® has used as first-line therapy of uncomplicated Plasmodium falciparum malaria in Ethiopia since it replaced sulfadoxine-pyrimethamine in 2004. Studies done in different parts of Asia showed P. falciparum resistance against artemisinin-based therapies [4]. Furthermore, a report from Ethiopia indicated low-level resistance of P. falciparum to Coartem® treatment [5]. Therefore, the aim of this study was to investigate the therapeutic efficacy of Coartem® in the treatment of uncomplicated P. falciparum malaria in Woreta Town, South Gonder Zone, Ethiopia.
The study was conducted between November 2010 and January 2011 in Woreta Town, South Gonder Zone, Ethiopia. It is located in the Debub Gondar Zone of the Amhara Region, east of Lake Tana and south of Addis Zemen, with an elevation of 1828 meters above sea level [6].
The study was conducted based on the revised WHO recommendation for the assessment and monitoring of antimalarial drug efficacy [7]. According to the WHO protocol for estimating the population proportion, a minimum sample size of 73 patients is required. This calculation is based on expected clinical failures of 5%, 95% confidence interval, and 5% precision.
The formula was used for the calculation .
Therefore, once the minimum number of the sample obtained, by adding 20% contingency, (73 + 15), 88 patients enrolled for the study.
Blood films were taken at least eight times for each study participants during the study period (day 0, 1, 2, 3, 7, 14, 21 and 28) and on any unexpected visit. Giemsa stained thick and thin blood smears were prepared on the same slide from each study participants in each follow-up day. Thin smears were used for species identification and thick smear used for determination of parasite density. The slides were examined under a light microscope using 100x oil immersion objective lens.
The number of parasites per microliter of blood was calculated using the formula:
Enrolled patients were treated with the standard six dose regimen of Coartem, given twice daily for three consecutive days. The tablets were administered at 0, 8, 24, 36, 48 and 60 hours. Dosing was administered according to the WHO weight-based guideline [7].
Coartem was given based on the weight on days 0, 1, and 2. Follow up was done on days; 1, 2, 3, 7, 14 21 and 28. At all visits, their health condition was assessed and axillary temperature and the blood sample was taken for parasitological assessment. Haemoglobin level was measured on days 0, 14 and 28.
Axillary temperature was measured at all visits using a digital thermometer. Any measured temperature below 36 °C was repeated.
Finger-prick blood samples were collected from each patient on day 0 for determination of anaemia among the study participants. Furthermore, haemoglobin level was measured on day 14 and 28 to assess the recovery of anaemia, as follows. The fingertip was cleaned with alcohol and prinked with sterile lancet wiped away the first drop. Then, the next drop of blood was used to fill the microcuvette. Finally, the micro cuvette was pushed into haemocue analyzer and the displayed value was recorded in g/dl.
Early treatment failure (ETF): Presence of any of the following: Development of danger signs or severe malaria on day 1, 2, or 3 in the presence of parasitemia; Parasitaemia on day 2 higher than day 0 count irrespective of axillary temperature; Parasitaemia on day 3 with axillary temperature ≥ 37.5 °C; Parasitaemia on day 3, ≥ 25% of count on day 0.
Late clinical failure (LCF): The presence of any of the following: Development of danger signs or severe malaria after day 3 in the presence of parasitemia without previously meeting any of the criteria of ETF; the presence of parasitemia and axillary temperature ≥ 37.5 °C or history of fever on any day from day 4 to day 28 without previously meeting any of the criteria of ETF.
Late Parasitological Failure (LPF): Presence of any of the following: The presence of parasitemia on any day from day 7 to day 28 and irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure or late clinical failure.
Adequate clinical and parasitological response (ACPR): Presence of any of the following:- The absence of parasitemia on day 28 irrespective of axillary temperature without previously meeting any of the criteria of early treatment failure, late clinical failure, or late parasitological failure.
Ethical clearance was obtained from Addis Ababa University and written informed consent was obtained from each participant or guardians of those under 18-years-old.
Data of patients excluded from the study due to protocol violation and lost to follow up were excluded from treatment outcome analysis. Data were double entered in the WHO excel spreadsheet designed for therapeutic efficacy data. Paired T-test was used to compare haemoglobin changes from baseline to day 14 and day 28 and a two-sided p-value < 0.05 was considered statistically significant. Microsoft Excel was used to show parasite and fever clearance following Coartem treatment.
A baseline characteristic of the study participants was shown in Table 1. During the enrollment period (between November 2010 and January 2011), a total of 2240 blood films of patients suspected to have malaria were screened for eligibility in Woreta health center and of this, 88 with confirmed falciparum malaria were enrolled and treated with Coartem®. Age stratification showed a large number of the study population (65%, n = 57) were adults (> 15 years), 19% were between 5 and 15 years and the remaining 12% were children under five years. The average parasite count was 14,362/µl (500-85,000) and the highest mean parasite count (23,788) was observed in children under five years as compared with other age groups. The mean haemoglobin concentration was 13 g/dl (6.7-18.7). Of the 88 study participants, 60 were febrile at day 0 and 30.7% were anaemic. About 58% of children under five years, 29.4% of children between 5 and 15 years and 33.3% of adults were anaemic at the day of admission. The average axillary temperature was 38.4 °C. The average weight of the study participants was 26.6 Kg (10.5 Kg-70 Kg). Although information on bed net utilization collected from enrolled patients in the study showed a good record of bed net distribution (78.4%), some patients reported that they don’t use bed nets regularly.
Table 1: Baseline characteristics of uncomplicated P. falciparum malaria patients enrolled in the in vivo efficacy study of coartem in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011. View Table 1
Treatment resulted in rapid clearance of parasites. Parasites were cleared from 72.7% patients by day 1 and only one patient remained positive till day 3 (Figure 1).
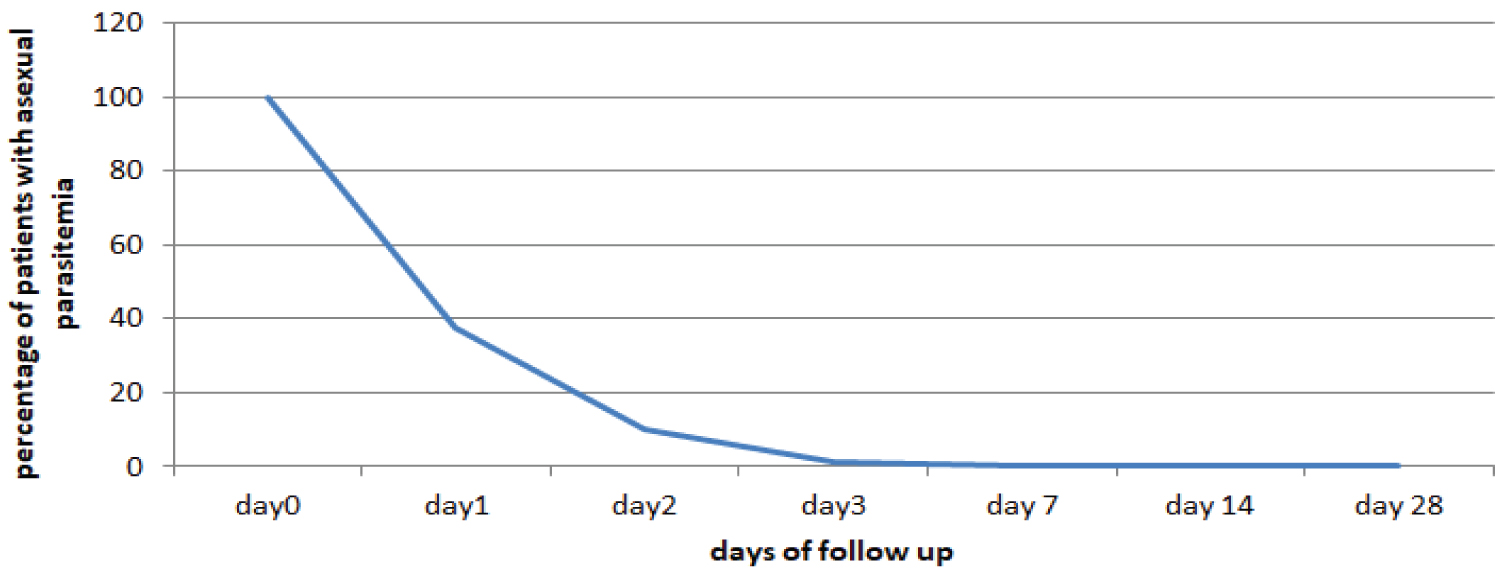 Figure 1: Percentage of uncomplicated P. falciparum patients with asexual parasitemia following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
View Figure 1
Figure 1: Percentage of uncomplicated P. falciparum patients with asexual parasitemia following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
View Figure 1
Fever clearance was also rapid. Fever was cleared from almost all patients on day 3, (Figure 2).
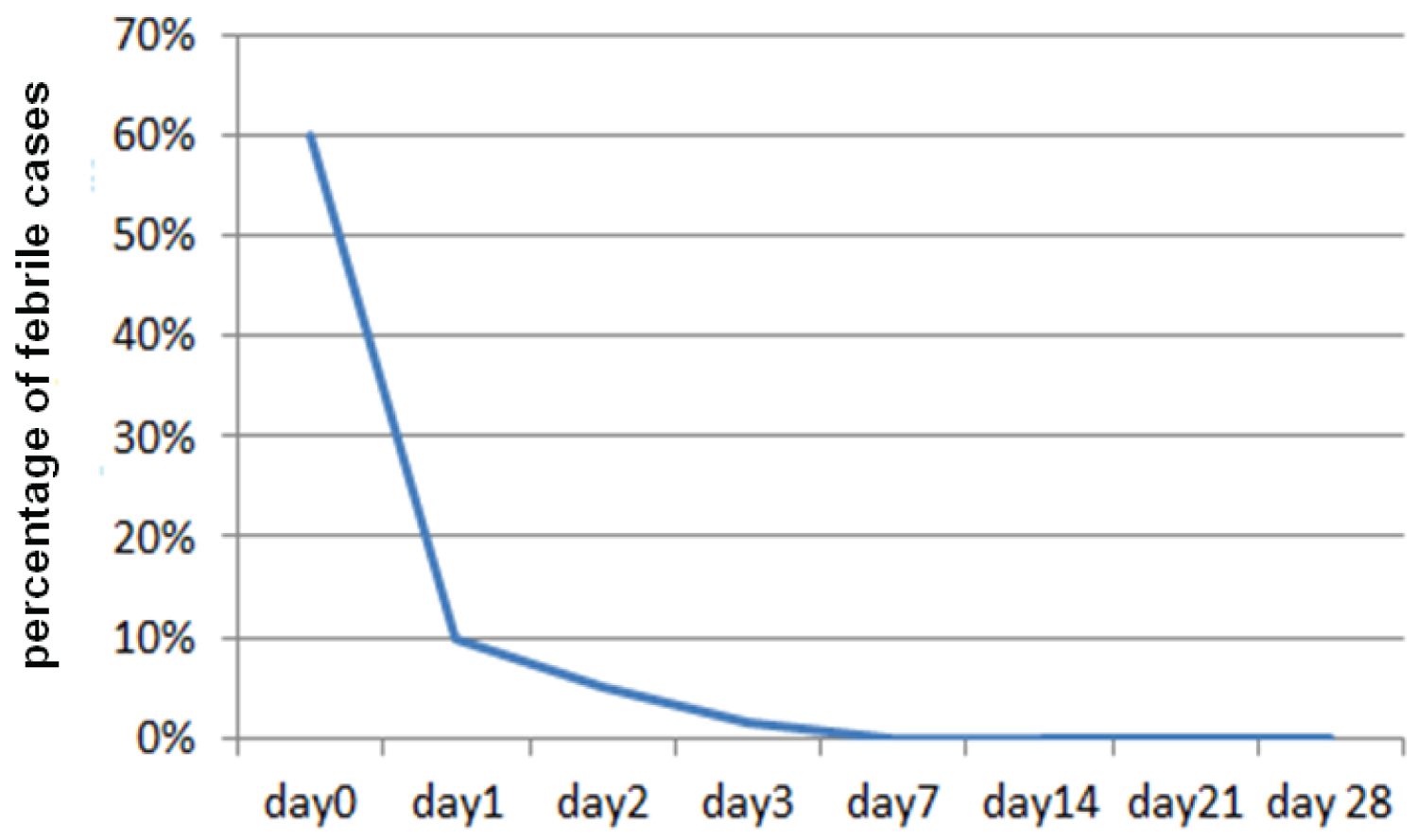 Figure 2: Percentage of uncomplicated P. falciparum malaria patients with fever following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
View Figure 2
Figure 2: Percentage of uncomplicated P. falciparum malaria patients with fever following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011.
View Figure 2
Of the 88 study participants, ten were excluded from treatment outcome analysis due to protocol violation and lost to follow up. As a result, 78 subjects were included in the treatment outcome analysis. Coartem showed 100% adequate clinical and parasitological response (Table 2).
Table 2: Cure rate of coartem against uncomplicated P. falciparum patients in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011. View Table 2
A significant decrease in haemoglobin concentration (from 13 g/dl to 12.6% g/dl) was observed on day 14 following treatment, with the resultant increase in the proportion of anaemic cases from 30.7% on day zero to 36.1% on day 14. However, a significant increase in haemoglobin concentration observed at day 28 (from 13.0 g/dl in day 0 to 13.4 in day 28), with the resultant decline in anaemia prevalence (Table 3).
Table 3: Change in haemoglobin concentration in Coartem treated uncomplicated P. falciparum patients in Woreta Health Center, Woreta Town, South Gonder Zone, Ethiopia, November 2010 to January 2011. View Table 3
There is no serious side effect observed following Coartem treatment throughout the 28-days follow up period (Table 4). Coartem is safe and well tolerated for the treatment of uncomplicated P. falciparum malaria in the study area.
Table 4: adverse events experienced by the uncomplicated P. falciparum malaria study participants following Coartem treatment in Woreta Health Center, Woreta Town, South Gonder Zone, Amhara region, Ethiopia. View Table 4
The 100% adequate clinical and parasitological response observed in the present study is consistent with the result of studies conducted in different parts of Ethiopia [8,9]. However, it is contrary to findings from Asia and other studies carried out in two regions of Ethiopia, which reported a low level of Coartem treatment failure [4,5,10]. The low level of treatment failure in these areas might be due to the evolution of Coartem resistant strains of P. falciparum.
The artemisinin derivatives are fast-acting on different blood stages of the parasite [11], which explains the short time required for clearance of parasitemia. In agreement with this study, studies from different parts of Ethiopia [5,8-10], demonstrated rapid clearance of parasitemia in coartem treated uncomplicated P. falciparum malaria patients.
The rapid clearance of fever noted in this study is consistent with findings of other studies in Ethiopia [5,8-10]. The fact that fever is attributed to various cytokines released in response to malaria toxins and various metabolic end products released into the bloodstream following the breakdown of infected RBCs at the completion of schizogony [12], could explain the strong association between fever clearance and parasite clearance following Coartem treatment.
The fact that anaemia is a result of haemolysis of RBCs following erythrocytic-schizogony [13], may explain the strong relationship between clearance of asexual parasitemia and recovery of anaemia following Coartem treatment. The significant improvement in haemoglobin level following Coartem treatment noted in this study is an agreement with findings of studies done in different parts of Ethiopia [8,9].
The good safety profile of Coartem observed in this study is consistant with other researchers that reported coartem as safe and well tolerated for the treatment of uncomplicated P. falciparum malaria in other study areas [8,14].
From this study, it can be conclude that Coartem® results in rapid clearance of fever and parasitemia; it achieves a high cure rate and increases haemoglobin level on day 28 post-treatment. Coartem is safe and well tolerated for the treatment of uncomplicated P. falciparum malaria in the study area.
As the parasite may develop resistance for the drug, further study on therapeutic efficacy of Coartem® in treatment of uncomplicated P. falciparum in Wortea town will be important.
This work was supported by the Ethiopian Health and Nutrition Research Institute.
There is no conflict of interest.
Data used to support the conclusions of this study are included in the article.
I am grateful for Professor Beyene Petrose; Mr. Moges Kassa, Mr. Ashenafi Assefa, and Mr. Hussen for their supervision of the research. I am also grateful for Mr. Abrheham Fasil (clinician) and Miss Kassanesh (laboratory technician) for their involvement in the fieldwork. I would like also to acknowledge the study participants and staff members of Wortea health center for their contribution during screening and follow up the study participants.