Department of Biochemistry, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia (UPM), Malaysia
Abstract
Heavy metals emission, in particular, mercury is ever increasing due to global urbanization and industrialization. Due to increasing number of health problems related to heavy metals contamination, monitoring it becomes a crucial task for authorities and environmentalists. Therefore, the development of a nanobiosensing technique that can detect mercury ions as low as 1 ppb for its limit of quantification is necessary to provide sufficient information to ensure a sustainable and healthy environment for the community around the world. Herein we reviewed reported studies on the mercury ions bioavailability and recent progress of its detection methods. Comparison of detection methods based on electrochemical and optical technique has been made. There are two main technological gaps that need to be filled, which are sensitivity and practicality of measurement at point-of-check for in-situ analysis.
Introduction
Heavy metals are non-biodegradable elements that fall in a group of natural constituents of the earth. Their specific gravity is greater than 5.0, and it has relatively high atomic weight [1,2]. Many industrial applications nowadays involve the use of heavy metals for their production which includes mining activities (Cu, Cd, Co, Cr, Ni, Pb, Zn, Hg and As) [3-6], power generation industries (As, Cd, Pb and Hg) [7], smelting industries (Cd, Pb, Cu and Zn) [8,9] battery production (Pb, As, Cd, Ni, Hg) [10], dyes and textile manufacturing industries (Al, Co, Ni, TI, Pb, Cd, Zn, Cu, Cr, Fe and Mn) [11,12], biosolids and manure (As, Cd, Cr, Cu, Pb, Hg, Ni, Se, Mo, Zn, Tl, Sb) [13], as well as fertilizer production (As, Cd, Pb, Cu, Cr and Zn) [14-16], and other applications. Artisanal and small-scale gold mining that use amalgamation as the gold extraction technique, for example, is rampant across Africa and South America. This sector alone is estimated to produce 1000 tonnes of Mercury per annum [17]. If these industries are not tightly regulated, the level of heavy metals will be elevated tremendously in the environment and can cause severe problems to the biosphere [17,18]. Accumulation of heavy metal contaminants can be found in water, sludge, air, and soil. Indirect ingesting of these metals will cause bioaccumulation in living organisms and eventually will lead to biomagnification, a phenomenon of metal ions intensification in higher trophic levels [19,20].
A small amount of heavy metals including Arsenic, Chromium, and Cadmium have preferential metabolic functions. Obviously, however, it can be detrimental if it exceeds the maximum permissible limit (MPL) that is also known as the toxic dosage [21-25]. Lead, for example, is often associated with damage to the human kidney [26,27] and central nervous system [28,29]. It can also cause anemia [30], hypertension [31,32], immunotoxicity, and oxidative stress [33]. Very recently, it is suggested that the presence of lead causes a sympathovagal imbalance that leads to a high blood pressure [34]. Copper is another well-known heavy metal that is essential for human metabolism activities. Its hemeostasis is maintained by CsoR protein [35], and in the form of ceruloplasmin it aids the transport of iron to the cells [36,37]. Overexposure to this element, however, will lead to dermal toxicity [38], respiratory problem [39] and can lead to kidney damage through the up-regulation of tumor gene suppressor p53 and apoptotic gene caspase-3 [40]. Mercury, one of the top ten elements or chemicals that pose concerns in public health, is known as neurotoxicant. Exposure to a low level of Mercury can cause neurological and behavioral disorder [41], heart disease [42], and permanent damage to vital organs. Exposure to > 1.6 ppb (or μg/kg body weight) and > 4.0 ppb of mercury can cause neurocognitive effects and kidney damages, respectively [43,44]. This is even lower toxic dosage compared to As, Cd, and Pb for similar adverse effects.
Heavy metal exposure not only affects human health but it also can disrupt an ecosystem. In the open ocean, 99% of plastic debris that pollutants like mercury are clinging onto is untraceable [45]. The estuarine biota is often in danger as industrial discharges from factories is usually released into the nearby river. In a recent study, neurotoxic MeHg was found to be bioaccumulated in the pelagic food web, in which will become human food sources later on [46]. When this contaminated food is consumed, it will result in food poisoning among the walks of life on earth. The consumption of contaminated food is a major pathway of human exposure to heavy metals as compared to inhalation and dermal contact.
Among all heavy metals, mercury is the most detrimental due to its neurotoxic and nephrotoxic properties. Mercury was started to be used as separating agent to separate fibers of fur from the pelt in a felting industry, and as a preservative for seeds and vaccine. Recent data shows that inorganic mercury was excreted as waste from coal-burning power plant, waste incinerators, alkali-chlor factory and gold mining industry. Human exposure to mercury can occur via eating MeHg-contaminated food (fish, shellfish, and other aquatic life), dental amalgam procedure, usage of inorganic mercury products (medication, germicidal soap and skin cream), occupational exposure (mining or mercury-related industry) and usage of any other mercury-based products (thermometer, sphygmomanometer, fluorescent light bulbs and batteries).
Mercury is commonly bioavailable (Figure 1) and enters the food web as methylmercury (MeHg) which can be magnified as it passes up the food chain. However, the natural form of mercury that often excreted out from the industrial area is inorganic mercury, mercury (II) ions, and elemental mercury. Elemental mercury, Hg0 exist in the form of volatile liquid from soil-air flux and other anthropogenic releases that can remain suspended in the atmosphere for one to two years long [47,48]. It can be converted to Hg2+ via oxidation and eventually will reside in the water sources or soil through the rain. This Hg2+ will then be converted to methylmercury through methylation process by anaerobic sulfur reduction bacteria which includes Desulfovibrio desulfuricans, Desulfobulbus propionicus, Desulfococcus multivorans, Desulfobacter sp., and Desulfobacterium sp. [49].
The abundance of mercury in the environment is inevitable since the natural ecosystem produces it as well [50]. The concentration of Mercury in the ocean, precisely at the level shallower than 100 m was tripled from its pre-anthropogenic condition to 0.6 pM [51]. The increment of their concentration especially when localized raised concern among environmentalists and population as a whole. According to the World Health Organization (WHO), the MPL for almost all heavy metals in the environment must be below 0.2 ppm. Further, the MPL of mercury ions, have to be below 0.002 ppm.
Due to increasing industrial and mining activity globally, we are now facing a silent threat from mercury contamination. The anthropogenic release of mercury was estimated at 2320 tonnes/year in 2010 [52]. Meanwhile the United Nations Environmental Programme (UNEP) and the Arctic Monitoring and Assessment Programme (AMAP) approximated the anthropogenic mercury emissions globally has an uncertainty range of 1010-4070 tonnes/year. Artisanal and small-scale gold mining (ASGM) were identified as the major anthropogenic sources (37%) and the dental amalgam emitted through cremation were identified as the minor anthropogenic sources (0.2%) [53]. Figure 2 below shows a mercury emission percentage based on different sources of emission.
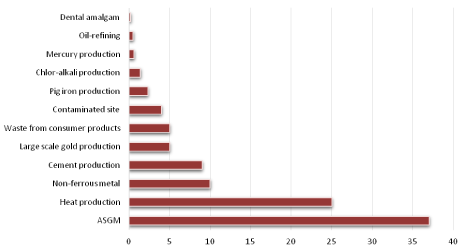 Figure 2: Mercury emission percentage based on different sources of emission. View Figure 2
Figure 2: Mercury emission percentage based on different sources of emission. View Figure 2
There are several cases worth mentioning to show that contaminated water is one of the main sources where mercury contamination could affect most lives. Water sources are the reservoir where all the inorganic mercury would reside before it will be methylated and would be magnified in the higher organism hierarchy as it passes up a food chain. In general population, dietary intake is the most common pathway for mercury contamination. The classic case of Minamata Bay Japan, in 1953 witnessed 2252 victims poisoned with MeHg (5.61 ppm to 35.7 ppm) in their marine product [54]. In 1964, the similar case happened in Niigata with approximately 700 victims through the same exposure of the contaminated marine products [37]. In 2006, China faced a threat from mercury pollution due to their mining and coal-burning industry. National Bureau of Oceanography (NBO) of China stated that there is approximately 77 ton of Hg were released into the coastal area through river annually [56,57].
Development of Nanosensors to Detect Heavy Metals
Conventional methods
The determination of heavy metals in the environment is essential in order to find a targeted area for remediation process [58-60]. Several detection techniques have been developed and commercially available, including atomic absorption spectrometry (AAS) [61-63], Synchrotron X-ray fluorescence (SXRF) spectrometry [64], microwave plasma atomic emission spectroscopy (MP-AES) [65,66], inductively coupled plasma mass spectrometry (ICP-MS) [67,68], and cold vapour atomic fluorescence spectrometry (CVASF) [69] and thermal decomposition mercury analyser (TDMA) [70]. These techniques provide sensitive and accurate result in determining the level of heavy metal in the environment. Most of these techniques, however, require a sophisticated machine with the time-consuming procedure, and generally, need expensive consumables which make it non-practical to be used for in-situ analysis at a mass and constant screening level. It is also worthy to note that digestion step under an extreme condition is usually required for most of these techniques to free the metal ions prior to the measurement. This makes the results to be questionable because the original environmental condition of those metals is disregarded [71]. These limitations lead to the needs of developing simple yet effective techniques to detect heavy metal contamination at the point-of-check of an environment. This is especially important and useful to be used as a front-line screening procedure.
Biosensing methods
In recent years, researchers have turned their attention towards biosensor techniques to be used to detect heavy metal contamination. Biosensors are a device that uses any biological based elements including protein, enzymes, cells and nucleic acids to detect the presence of molecules; in this case, metal ions. It has two main components; biological element as a receptor or capturing agent, and physicochemical element as a transducer [72]. In the middle between these two components lay a surface chemistry that plays an important role at the interfacial. The transduced signal of a biosensing method can be amplified, enhanced and more effective by manipulating the surface chemical properties of a particular sensing platform. The biorecognition elements typically are whole cells, enzymes, antibodies, peptides, small molecules, or nucleic acids that could react selectively and sensitively with the target analytes. Meanwhile, signal transduction converts the biological response resulting from the interaction with the target analytes into a distinct and quantifiable signal [73]. Ionic elements like Hg2+ has at least two characteristics that can be leveraged as a sensing counterpart element; i) As a charge-bearing atom, electrochemical sensing principle can be used because its presence will alter current or impedance. ii) Affinity towards capturing agents can be used as a chemical switch to generate molecular structural transfiguration that subsequently produces an optical sensing signal. We tabulated some of the newly developed biosensing methods that utilized these two concepts (Table 1). The electrochemical-based biosensor has the advantage for the sensitivity, whereas the optical-based biosensor can easily be miniaturized, primarily through the usage of color recognition apps on the smartphone.
Table 1: Detection of mercury ions, Hg2+ using optical-based or electrochemical-based biosensor. View Table 1
Capturing agents
In environmental monitoring, most biosensors have been developed for detection of pesticides and heavy metals due to their occurrence in the environment [74]. Some groups have reported using whole cell [75], microorganisms such as bacteria, and algae to detect heavy metal ions [76]. Alternatively, biomolecules can be utilized as effective capturing agents. Tanaka, et al. utilized thymine-thymine (T-T) mismatches to capture mercury ions effectively at [77]. They showed that mercury ion out of all ions has a greater binding affinity with thymine mismatches and suggesting that that Hg2+ is a substitute for imino group and bind with the nitrogen of the thymine residue. Soft acidic metals like mercury have a strong affinity with sulfur, nitrogen and oxygen elements. Hence protein antibody has become a good capturing agent to establish a new biosensing platform [78]. In addition to that, monoclonal antibodies have been generated to specifically recognize heavy metals ions such as Hg2+, Cd2+, Cu2+, Ni2+, Pb2+, Co2+, and Ag+ [79-82]. Chelating agents like Rhodamine- [83-87], calix[4]arene- [87], thioether- [88], and aniline-derivative can be used to specifically capture mercury at relatively high affinity in-vitro or in-vivo (Table 2). Moreover, oxygen, nitrogen, or sulfur-based crown structures also exhibit effective mercury chelating [89,90]. These strong binding kinetics often incorporated with fluorophores in the Förster resonance energy transfer (FRET) techniques using terphenyl derivatives or any other branched moiety as a flexible linker. An electrochemical method can also be employed by integrating rhodamine with ferrocene substituent [86]. Spirolactam ring opening in rhodamine can also be manipulated to induce a color change of the rhodamine solution. Hence with the presence and absence of Hg2+, the chemosensor can be turned on and off, respectively. There are also structure-switching DNA biosensors that are formed through formation of many weak non-covalent bonds which can detect mercury ions as good as CVAFS with an only minimal volume of sample is required for the detection purpose [71,91]. Yu & Tseng that used 3-mercaptopropionic acid and adenosine monophosphate to detect mercury ions down to 0.1 ppm in a high salt condition.
Table 2: Capturing agents that have been used to capture mercury (II) ions. View Table 2
Colorimetric Assay as a Biosensor to Detect Mercury Ions
Simple, robust, and inexpensive biosensors for detecting and monitoring pollutants in the environment can be developed using colorimetric assay method. Due to its vivid visible response and rapid measurement process, colorimetric biosensors have been utilized to detect mercuric [92,93], and other metal ions [76]. Since the signal produced in the form of bright and distinctive color, thus, it can act as an indicator to provide an early warning for detection and monitoring of the level of heavy metals in the environment.
Gold-nanoparticles (AuNPs)-based biosensor is another type of versatile colourimetric assay methods. It has high extinction coefficient which makes it suitable to act as a sensing platform [78,94,95]. Gold-nanoparticles possess an exploitable property due to its intrinsic surface plasmon resonance that strongly depends on the sizes of the particles. It is also possible to modify the AuNPs with various kinds surface functionalization to specifically serve as mercury ions capturing agent in sub-ppm concentration [96]. Moreover, the color produced by the AuNPs is various, depending on the distance between the particles. As the distance between particles decreases, it will promote plasmon coupling which will shift the plasmon-band to a lower energy level. This will result in a change of color of the AuNPs solution from red to blue (in the case of 20 nm and 40 nm); namely a redshift phenomenon [97]. AuNPs can aggregate and disperse, which can be regulated by the ligand that is functionalized onto its surfaces. For example, the analyte that can bind with the ligand on top of the AuNPs will make two or more particles to attract each other which result in a subsequent change of color. The concept is also rightly applicable vice versa. The capability of gold nanoparticles to be conjugated with different types of ligands through surface chemistry, open a room of exploitation for this biosensing platform.
Bio-Conjugated Gold-Nanoparticles
Bio-conjugated gold nanoparticle is a modification onto gold nanoparticles surface in order to make it selective and sensitive towards desired interactions. Due to the capability of gold-nanoparticles to be conjugated with many biomolecules such as DNA, protein, and enzyme, there were many studies carried out to improve utilization of AuNPs [78]. There are three ways to conjugate and functionalize gold nanoparticles. The most common approach is via covalent coupling between gold and thiol group to ligate molecules onto the gold-nanoparticles surface [98]. There is also physical absorption where electrostatic or hydrophobic interactions are used. However, since this is a weak interaction, any physiological change to the AuNPs solution including pH and temperature can lead to detachment of ligand molecules [99]. The third approach is using an explicit specificity of ligand molecules by tagging gold-nanoparticles with antigen and uses it for detection of antibody. This approach is a fundamental idea to build a platform to detect any antibody and widely used in medical field. Hung, et al. develop a biosensor using series of alkanethiols which capable of detecting mercuric (Hg2+), silver (Ag+) and lead (Pb2+) ions [100]. They used sulfur group in alkanethiols to bind strongly with gold-nanoparticles and let the -OH functional group attract mercury ions.
Conclusion
The optical-based sensor is a promising sensor to detect mercuric pollution due to its vivid signal that is directly observable through the naked eye. The qualitative signal can be analyzed quantitatively using colour recognition and processing apps that are commercially available, and in-situ detection can be done through a smartphone. Furthermore, with the availability of the internet-of-things and cloud server, polluted areas and regions can be mapped in a real-time manner. Hence an automated early warning system can be put in operation [101]. Nonetheless, the reported studies shown here suggest that the sensitivity of optical-based detection methods remains a challenge. Electronic and electrochemical sensors, on the other hand, are very sensitive methods. However, to make a practical POC device, the electrochemical devices that are currently used need to be miniaturized, and enabled the operation without a plug-in electrical current source. The combination of the ability to detect mercury in a dynamic range of a very low concentration (ppt-ppb or pM-nM), and the POC compatibilities that allow in-situ analysis for mass screening, can open a new avenue of applications.
Acknowledgement
The authors would like to thank the Universiti Putra Malaysia for the support of this study under the project number UPM/700-2/1/GP-IPS/2016/9508300. The authors also thank Dr. Asilah Ahmad Tajudin and Dato' Dr. Abu Bakar for their useful discussions.
References
-
Duffus JH (2002) "Heavy metals"-a meaningless term? (IUPAC Technical Report). Pure Appl Chem 74: 793-807.
-
(1846) Illustrations of chemistry. Sci Am 1: 2-2.
-
García-Ordiales E, Esbrí JM, Covelli S, López-Berdonces MA, Higueras PL, et al. (2016) Heavy metal contamination in sediments of an artificial reservoir impacted by long-term mining activity in the Almadné mercury district (Spain). Environ Sci Pollut Res Int 23: 6024-6038.
-
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: A review. Environ Chem Lett 8: 199-216.
-
Garcia-Sanchez A, Alvarez-Ayuso E (2003) Arsenic in soils and waters and its relation to geology and mining activities (Salamanca Province, Spain). J Geochem Explor 80: 69-79.
-
Shallari S, Schwartz C, Hasko A, Morel JL (1998) Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci Total Environ 209: 133-142.
-
Pacyna JM (2009) Atmospheric emissions of arsenic, cadmium, lead and mercury from high temperature processes in power generation and industry. In: John Wiley & Sons, New York, USA, 69-87.
-
Zhang X, Yang L, Li Y, Li H, Wang W, et al. (2012) Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ Monit Assess 184: 2261-2273.
-
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Notices 2011: 20.
-
Orisakwe OE, Asomugha R, Afonne OJ, Anisi CN, Obi E, et al. (2004) Impact of effluents from a car battery manufacturing plant in Nigeria on water, soil, and food qualities. Arch Environ Health 59: 31-36.
-
Sungur Ş, Gülmez F (2015) Determination of metal contents of various fibers used in textile industry by MP-AES. Journal of Spectroscopy 2015: 5.
-
Islam MM, Halim MA, Safiullah S, Waliul Hoque SAM, Saiful Islam M (2009) Heavy Metal (Pb, Cd, Zn, Cu, Cr, Fe and Mn) content in textile sludge in Gazipur, Bangladesh. Res J Environ Sci 3: 311-315.
-
Basta NT, Ryan JA, Chaney RL (2005) Trace element chemistry in residual-treated soil: Key concepts and metal bioavailability. J Environ Qual 34: 49-63.
-
Atafar Z, Mesdaghinia A, Nouri J, Homaee M, Yunesian M, et al. (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160: 83-89.
-
Sabiha-Javied, Mehmood T, Chaudhry MM, Tufail M, Irfan N (2009) Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchem J 91: 94-99.
-
Mortvedt JJ (1995) Heavy metal contaminants in inorganic and organic fertilizers. Fertil Res 43: 55-61.
-
Spiegel SJ, Veiga MM (2010) International guidelines on mercury management in small-scale gold mining. J Clean Prod 18: 375-385.
-
Hilson G (2006) Abatement of mercury pollution in the small-scale gold mining industry: Restructuring the policy and research agendas. Sci Total Environ 362: 1-14.
-
Malik RN, Hashmi MZ, Huma Y (2014) Heavy metal accumulation in edible fish species from Rawal Lake Reservoir, Pakistan. Environ Sci Pollut Res Int 21: 1188-1196.
-
Bellinger DC, Chen A, Lanphear BP (2017) Establishing and achieving national goals for preventing lead toxicity and exposure in children. JAMA Pediatr 171: 616-618.
-
Strydom C, Robinson C, Pretorius E, Whitcutt JM, Marx J, et al. (2006) The effect of selected metals on the central metabolic pathways in biology: A review. Water SA 32: 543-554.
-
Refaie FM, Esmat AY, Mohamed AF, Nour WHA (2009) Effect of chromium supplementation on the diabetes induced-oxidative stress in liver and brain of adult rats. Biometals 22: 1075-1087.
-
Chandurvelan R, Marsden ID, Gaw S, Glover CN (2017) Acute and sub-chronic effects of sub-lethal cadmium exposure on energy metabolism in the freshwater shrimp, Paratya curvirostris. Ecotoxicol Environ Saf 135: 60-67.
-
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, et al. (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int 23: 8244-8259.
-
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7: 60-72.
-
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: A review with recent updates. Interdiscip Toxicol 5: 47-58.
-
Nakata H, Nakayama SMM, Oroszlany B, Ikenaka Y, Mizukawa H, et al. (2017) Monitoring lead (Pb) pollution and identifying pb pollution sources in Japan using stable Pb isotope analysis with kidneys of wild rats. Int J Environ Res Public Health 14: 56.
-
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 126: 5-19.
-
Guariglia SR, Stansfield KH, McGlothan J, Guilarte TR (2016) Chronic early life lead (Pb2+) exposure alters presynaptic vesicle pools in hippocampal synapses. BMC Pharmacol Toxicol 17: 56.
-
Hsieh N-H, Chung S-H, Chen S-C, Chen W-Y, Cheng Y-H, et al. (2017) Anemia risk in relation to lead exposure in lead-related manufacturing. BMC Public Health 17: 389.
-
Toscano CM, Simões MR, Alonso MJ, Salaices M, Vassallo DV, et al. (2017) Sub-chronic lead exposure produces β1-adrenoceptor downregulation decreasing arterial pressure reactivity in rats. Life Sci 180: 93-101.
-
Almeida Lopes ACB de, Silbergeld EK, Navas-Acien A, Zamoiski R, Martins Jr ADC, et al. (2017) Association between blood lead and blood pressure: A population-based study in Brazilian adults. Environ Health 16: 27.
-
Batool Z, Yousafzai NA, Murad MS, Shahid S, Iqbal A (2017) Lead toxicity and evaluation of oxidative stress in humans. PSM Biol Res 2: 79-82.
-
Simões MR, Preti SC, Azevedo BF, Fiorim J, Freire DD, et al. (2017) Low-level chronic lead exposure impairs neural control of blood pressure and heart rate in rats. Cardiovasc Toxicol 17: 190-199.
-
Marcus SA, Sidiropoulos SW, Steinberg H, Talaat AM (2016) CsoR is essential for maintaining copper homeostasis in Mycobacterium tuberculosis. PLoS One 11: e0151816.
-
Prashanth L, Kattapagari KK, Chitturi RT, Baddam VRR, Prasad LK (2015) A review on role of essential trace elements in health and disease. J Dr NTR Univ Health Sci 4: 75-85.
-
Saha A, Karnik A, Sathawara N, Kulkarni P, Singh V (2010) Ceruloplasmin as a marker of occupational copper exposure. J Expo Sci Environ Epidemiol 18: 332-337.
-
Li H, Toh PZ, Tan JY, Zin MT, Lee CY, et al. (2016) Selected biomarkers revealed potential skin toxicity caused by certain copper compounds. Sci Rep 6: 37664.
-
Chen Z, Meng H, Xing G, Chen C, Zhao Y, et al. (2006) Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett 163: 109-120.
-
Siddiqui MA, Alhadlaq HA, Ahmad J, Al-Khedhairy AA, Musarrat J, et al. (2013) Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One 8: e69534.
-
Carocci A, Rovito N, Sinicropi MS, Genchi G (2014) Mercury toxicity and neurodegenerative effects. Rev Environ Contam Toxicol 229: 1-18.
-
Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalano A (2017) Mercury exposure and heart diseases. Int J Environ Res Public Health 14: 74.
-
Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, et al. (2012) Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120: 799-806.
-
De Roma A, Esposito M, Chiaravalle E, Miedico O, De Filippis SP, et al. (2017) Occurrence of cadmium, lead, mercury, and arsenic in prepared meals in Italy: Potential relevance for intake assessment. J Food Compos Anal 63: 28-33.
-
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Úbeda B, et al. (2014) Plastic debris in the open ocean. Proc Natl Acad Sci U S A 111: 10239-10244.
-
Jonsson S, Andersson A, Nilsson MB, Skyllberg U, Lundberg E, et al. (2017) Terrestrial discharges mediate trophic shifts and enhance methyl mercury accumulation in estuarine biota. Sci Adv 3: e1601239.
-
Eckley CS, Parsons MT, Mintz R, Lapalme M, Mazur M, et al. (2013) Impact of closing canada's largest point-source of mercury emissions on local atmospheric mercury concentrations. Environ Sci Technol 47: 10339-10348.
-
Eckley CS, Blanchard P, McLennan D, Mintz R, Sekela M (2015) Soil-Air mercury flux near a large industrial emission source before and after closure (Flin Flon, Manitoba, Canada). Environ Sci Technol 49: 9750-9757.
-
King JK, Kostka JE, Frischer ME, Saunders FM (2000) Sulfate-Reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol 66: 2430-2437.
-
Selin NE (2009) Global biogeochemical cycling of mercury: A review. Annu Rev Environ Resour 34: 43-63.
-
Lamborg CH, Hammerschmidt CR, Bowman KL, Swarr GJ, Munson KM, et al. (2004) A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 512: 65-68.
-
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, et al. (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10: 5951-5964.
-
UNEP (2013) Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. UNEP Chemicals Branch, Geneva, Switzerland.
-
Harada M (1995) Minamata disease: Methyl mercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25: 1-24.
-
Maruyama K, Yorifuji T, Tsuda T, Sekikawa T, Nakadaira H, et al. (2012) Methyl mercury exposure at niigata, Japan: Results of neurological examinations of 103 adults. BioMed Research International 2012: 635075.
-
Jiang, Gui-Bin Shi, Jian-Bo Feng X-B (2006) MERCURY in China. Environ Sci Technol 12: 3673-3678.
-
Li P, Feng XB, Qiu GL, Shang LH, Li ZG (2009) Mercury pollution in Asia: A review of the contaminated sites. J Hazard Mater 168: 591-601.
-
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals-concepts and applications. Chemosphere 91: 869-881.
-
Omichinski JG (2007) Toward methyl mercury Bioremediation. Science 317: 205-206.
-
Melnick JG, Parkin G (2007) Cleaving mercury-alkyl bonds: A functional model for mercury detoxification by MerB. Science 317: 225-227.
-
Bannon DI, Chisolm JJ (2001) Anodic stripping voltammetry compared with graphite furnace atomic absorption spectrophotometry for blood lead analysis. Clin Chem 47: 1703-1704.
-
Fang Z, Růžička J, Hansen EH (1984) An efficient flow-injection system with on-line ion-exchange preconcentration for the determination of trace amounts of heavy metals by atomic absorption spectrometry. Anal Chim Acta 164: 23-39.
-
Maquieira A, Elmahadi HAM, Puchades R (1994) Immobilized Cyanobacteria for online trace metal enrichment by flow injection atomic absorption spectrometry. Anal Chem 66: 3632-3638.
-
Arai Y, Lanzirotti A, Sutton S, Davis JA, Sparks DL (2003) Arsenic speciation and reactivity in poultry litter. Environ Sci Technol 37: 4083-4090.
-
Poirier L, Nelson J, Gilleland G, Wall S, Berhane L, et al. (2017) Comparison of preparation methods for the determination of metals in petroleum fractions (1000 °F+) by microwave plasma atomic emission spectroscopy. Energy Fuels 31: 7809-7815.
-
Ríos SEG, Peñuela GA, Botero CMR (2017) Method validation for the determination of mercury, cadmium, lead, arsenic, copper, iron, and zinc in fish through microwave-induced plasma optical emission spectrometry (MIP OES). Food Anal Methods 10: 3407-3414.
-
Shih TT, Hsieh CC, Luo YT, Su YA, Chen PH, et al. (2016) A high-throughput solid-phase extraction microchip combined with inductively coupled plasma-mass spectrometry for rapid determination of trace heavy metals in natural water. Anal Chim Acta 916: 24-32.
-
Yamakawa A, Moriya K, Yoshinaga J (2017) Determination of isotopic composition of atmospheric mercury in urban-industrial and coastal regions of Chiba, Japan, using cold vapor multicollector inductively coupled plasma mass spectrometry. Chem Geol 448: 84-92.
-
Ebdon L, E Foulkes M, Roux SL, Muñoz-Olivas R (2002) Cold vapour atomic fluorescence spectrometry and gas chromatography-pyrolysis- atomic fluorescence spectrometry for routine determination of total and organometallic mercury in food samples. Analyst 127: 1108-1114.
-
Sasamoto T, Otani H, Hirayama I, Hayashi M, Baba I, et al. (2016) Dietary exposure to dioxins, polychlorinated biphenyls, and heavy metals in the tokyo metropolitan area from 1999 to 2014. In: Persistent organic chemicals in the environment: Status and trends in the pacific basin countries I contamination status. American Chemical Society 1243: 85-106.
-
Long F, Zhu A, Shi H, Wang H, Liu J (2013) Rapid on-site/in-situ detection of heavy metal ions in environmental water using a structure-switching DNA optical biosensor. Sci Rep 3: 2308.
-
Turner AP (2013) Biosensors: Sense and sensibility. Chem Soc Rev 42: 3184-3196.
-
Ripp S, Diclaudio ML, Sayler GS (2010) Biosensors as environmental monitors. Environ Microbiol 213-233.
-
Verma N, Singh M (2005) Biosensors for heavy metals. Biometals 18: 121-129.
-
Liu Q, Cai H, Xu Y, Xiao L, Yang M, et al. (2007) Detection of heavy metal toxicity using cardiac cell-based biosensor. Biosens Bioelectron 22: 3224-3229.
-
Zhai J, Yong D, Li J, Dong S (2012) A novel colorimetric biosensor for monitoring and detecting acute toxicity in water. Analyst 138: 702-707.
-
Tanaka Y, Oda S, Yamaguchi H, Kondo Y, Kojima C, et al. (2007) 15N-15N J-Coupling across HgII: Direct observation of Hgii-mediated T-T base pairs in a DNA duplex. J Am Chem Soc 129: 244-245.
-
Aragay G, Pons J, Merkoçi A (2011) Recent trends in Macro-, Micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev 111: 3433-3458.
-
Shu Q, Liu M, Ouyang H, Fu Z (2017) Label-free fluorescent immunoassay for Cu2+ ion detection based on UV degradation of immunocomplex and metal ion chelates. Nanoscale 9: 12302-12306.
-
Wylie DE, Lu D, Carlson LD, Carlson R, Babacan KF, et al. (1992) Monoclonal antibodies specific for mercuric ions. Proc Natl Acad Sci U S A 89: 4104-4108.
-
Blake DA, Chakrabarti P, Khosraviani M, Hatcher FM, Westhoff CM, et al. (1996) Metal binding properties of a monoclonal antibody directed toward metal-chelate complexes. J Biol Chem 271: 27677-27685.
-
Jones RM, Yu H, Delehanty JB, Blake DA (2002) Monoclonal antibodies that recognize minimal differences in the three-dimensional structures of metal-chelate complexes. Bioconjug Chem 13: 408-415.
-
Bhalla V, Tejpal R, Kumar M (2010) Rhodamine appended terphenyl: A reversible "off-on" fluorescent chemosensor for mercury ions. Sens Actuators B Chem 151: 180-185.
-
Ko SK, Yang YK, Tae J, Shin I (2006) In vivo monitoring of mercury ions using a rhodamine-based molecular probe. J Am Chem Soc 128: 14150-14155.
-
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2010) A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37: 1465-1472.
-
Yang H, Zhou Z, Huang K, Yu M, Li F, et al. (2007) Multisignaling optical-electrochemical sensor for Hg2+ based on a rhodamine derivative with a ferrocene unit. Org Lett 9: 4729-4732.
-
Othman AB, Lee JW, Wu JS, Kim JS, Abidi R, et al. (2007) Calix[4]arene-Based, Hg2+ -Induced Intramolecular Fluorescence Resonance Energy Transfer Chemosensor. J Org Chem 72: 7634-7640.
-
Nolan EM, Lippard SJ (2003) A "Turn-On" fluorescent sensor for the selective detection of mercuric ion in aqueous media. J Am Chem Soc 125: 14270-14271.
-
Yoon S, Albers AE, Wong AP, Chang CJ (2005) Screening mercury levels in fish with a selective fluorescent chemosensor. J Am Chem Soc 127: 16030-16031.
-
Gupta VK, Chandra S, Agarwal S (2003) Mercury selective electrochemical sensor based on a double armed crown ether as ionophore. IJC-A 42: 813-818.
-
Bhalla V, Tejpal R, Kumar M, Sethi A (2009) Terphenyl derivatives as "turn on" fluorescent sensors for mercury. Inorg Chem 48: 11677-11684.
-
Wang GL, Zhu XY, Jiao HJ, Dong YM, Li ZJ (2012) Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens Bioelectron 31: 337-342.
-
Yu CJ, Tseng WL (2010) Colorimetric detection of mercury(II) in a high-salinity solution using gold nanoparticles capped with 3-mercaptopropionate acid and adenosine monophosphate. Langmuir 24: 12717-12722.
-
Saha K, Agasti SS, Kim C, Li X, Rotello VM (2012) Gold nanoparticles in chemical and biological sensing. Chem Rev 112: 2739-2779.
-
Guo Y, Wang Z, Qu W, Shao H, Jiang X (2011) Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron 26: 4064-4069.
-
Knecht MR, Sethi M (2009) Bio-inspired colorimetric detection of Hg2+ and Pb2+ heavy metal ions using Au nanoparticles. Anal Bioanal Chem 394: 33-46.
-
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem Rev 107: 4797-4862.
-
Priyadarshini E, Pradhan N (2017) Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens Actuators B Chem 238: 888-902.
-
Tan J, Liu R, Wang W, Liu W, Tian Y, et al. (2010) Controllable aggregation and reversible pH sensitivity of AuNPs regulated by carboxymethyl cellulose. Langmuir 26: 2093-2098.
-
Hung YL, Hsiung TM, Chen YY, Huang YF, Huang CC (2010) Colorimetric detection of heavy metal ions using label-free gold nanoparticles and alkanethiols. J Phys Chem C 114: 16329-16334.
-
Wei Q, Nagi R, Sadeghi K, Feng S, Yan E, et al. (2014) Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano 8: 1121-1129.
-
Chai F, Wang C, Wang T, Ma Z, Su Z (2010) L-cysteine functionalized gold nanoparticles for the colorimetric detection of Hg2+ induced by ultraviolet light. Nanotechnology 21: 025501.
-
Darbha GK, Singh AK, Rai US, Yu E, Yu H, et al. (2010) Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J Am Chem Soc 130: 8038-8043.
-
Sudibya HG, He Q, Zhang H, Chen P (2011) Electrical detection of metal ions using field-effect transistors based on micropatterned reduced graphene oxide films. ACS Nano 5: 1990-1994.
-
Gong J, Zhou T, Song D, Zhang L, Hu X (2009) Stripping voltammetric detection of mercury (II) based on a bimetallic Au-Pt inorganic-organic hybrid nanocomposite modified glassy carbon electrode. Anal chem 82: 567-573.
-
Gong J, Zhou T, Song D, Zhang L (2010) Monodispersed Au nanoparticles decorated graphene as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury (II). Sensors and Actuators B: Chemical 150: 491-497.
-
Xu H, Zeng L, Xing S, Shi G, Xian Y, et al. (2010) Microwave-radiated synthesis of gold nanoparticles/carbon nanotubes composites and its application to voltammetric detection of trace mercury (II). Electrochemistry Communications 10: 1839-1843.
-
Xu RX, Yu XY, Gao C, Jiang YJ, Han DD, et al. (2013) Non-conductive nanomaterial enhanced electrochemical response in stripping voltammetry: The use of nanostructured magnesium silicate hollow spheres for heavy metal ions detection. Anal Chim Acta 790: 31-38.
-
Yu C, Guo Y, Liu H, Yan N, Xu Z, et al. (2013) Ultrasensitive and selective sensing of heavy metal ions with modified graphene. Chemical Communications 49: 6492-6494.
-
Yuan S, Peng D, Song D, Gong J (2013) Layered titanate nanosheets as an enhanced sensing platform for ultrasensitive stripping voltammetric detection of mercury (II). Sensors and Actuators B: Chemical 181: 432-438.
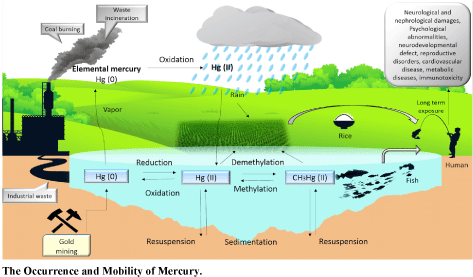 Figure 1:Mercury cycle schematic diagram. View Figure 1
Figure 1:Mercury cycle schematic diagram. View Figure 1
 Figure 2: Mercury emission percentage based on different sources of emission. View Figure 2
Figure 2: Mercury emission percentage based on different sources of emission. View Figure 2