Introduction: The expansion of Visceral Leishmaniasis in areas of recent emergency in the last two decades in Brazil can be understood by spatial analysis.
Methodology: Study of the spatial distribution of general VL and VL-HIV coinfection in the city of Bauru - SP, Brazil between 2003 to 2016. The cases were georeferenced and attributed to the Census sectors using demographic, socioeconomic and urban infrastructure predictors. The incidence in these sectors was analyzed in univariate and multivariate one-step Poisson regression models.
Results: The cumulative incidence rates of LV and LV-HIV (per 100,000 inhab.) In this period were 131.1 and 19.7, respectively. In the univariate analysis, the spatial distribution of VL and VL-HIV co-infection was negatively associated with per capita income, population density, paving and proportion of streets in sidewalks and rain drainage. In multivariable models, the incidence of VL was negatively associated with per capita income (Incident Rate Ratio [IRR], 0.89; 95% confidence interval [CI], 0.86-0.92) and population density (IRR, 94; 95% CI, 0.90-0.97), while co-infection was negatively associated with per capita income (IRR, 0.80; 95% CI, 0.71-0.91) and proportion of streets with rain drainage (TIR, 0.87; 95% CI, 0.78-0.98).
Conclusion: Demographic, socioeconomic and infrastructure deficits can influence the emergence patterns of VL in urban areas in developing countries.
Visceral leishmaniasis, Leishmania-HIV coinfection, Spatial epidemiology
Visceral Leishmaniasis (VL) is a relevant cause of morbidity and mortality in many developing countries [1]. In the past three decades, VL in Brazil changed from rural endemics of Northeastern States into an emergent disease of great urban centers [2]. The area affected spread southwards affecting São Paulo, the most populous state in the country. In this setting, the epidemics of VL and AIDS intertwined [3].
In urban areas of recent emergence, the spatial distribution of VL cases is not homogeneous [4]. Previous studies suggest that socioeconomic and environmental factors may shape de spatial distribution VL incidence [5]. Therefore, the study of those factors is likely to provide clues to determinants of the emergence of VL, ultimately pointing to areas/populations at risk and helping in directing preventive measures [6].
We studied the spatial distribution of overall VL and VL-HIV coinfection in the city of Bauru (340.000 inhabitants), inner São Paulo State, Brazil. That city is located at 22° 18' 55" S, 49° 3' 41"W, and at an altitude of 543 meters. The first notifications of VL in Bauru date from 2003. The cumulative incidence rates of VL and VL-HIV (per 100,000 inhabitants) in the period 2003-2016 were 131.1 and 19.7, respectively.
The addresses of subjects were georeferenced in QGIS 2.18 (QGIS Development Team [2016]. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org"), using UFT-8 codifications and MMQGIS geocodification algorithm. After georeferencing, cases were assigned to census sectors of the city of Bauru, Brazil, according to Brazilian Institute of Geography and Statistics (IBGE). Kernel density maps were generated in ArcGis 10.1 (ESRI, Redlands, CA) using the following algorithms: "integrate", "collect events" and "hotspot analysis".
The addresses of subjects were georeferenced in QGIS 2.18 (QGIS Development Team [2016]. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org"), using UFT-8 codifications and MMQGIS geocodification algorithm. After georeferencing, cases were assigned to census sectors of the city of Bauru, Brazil, according to Brazilian Institute of Geography and Statistics (IBGE). Kernel density maps were generated in ArcGis 10.1 (ESRI, Redlands, CA) using the following algorithms: "integrate", "collect events" and "hotspot analysis".
Census sectors were the units for analysis of predictors of VL and VL-HIV incidence. Variables included in the analysis were collected in the 2010 Brazilian Census Data (IBGE) and included and per capita income and population density. Other data collected from the same source included percentage of houses in places with sidewalks, paving of streets, drains for rainfall, trees, sewage and garbage in the open. Data were analyzed in Stata 14 (College Station, TX), using univariate and multivariable (single-step) models of Zero-inflated Poisson Regression.
Figure 1 presents spatial distribution of cases, in crude incidence per census sectors and Kernel densities. Predictors of geographic incidence are presented in Table 1. In multivariable models, overall VL incidence was negatively associated with population density and per capita income, while there was negative association of the incidence of VL-HIV coinfection with per capita income and presence of drains for rainfall.
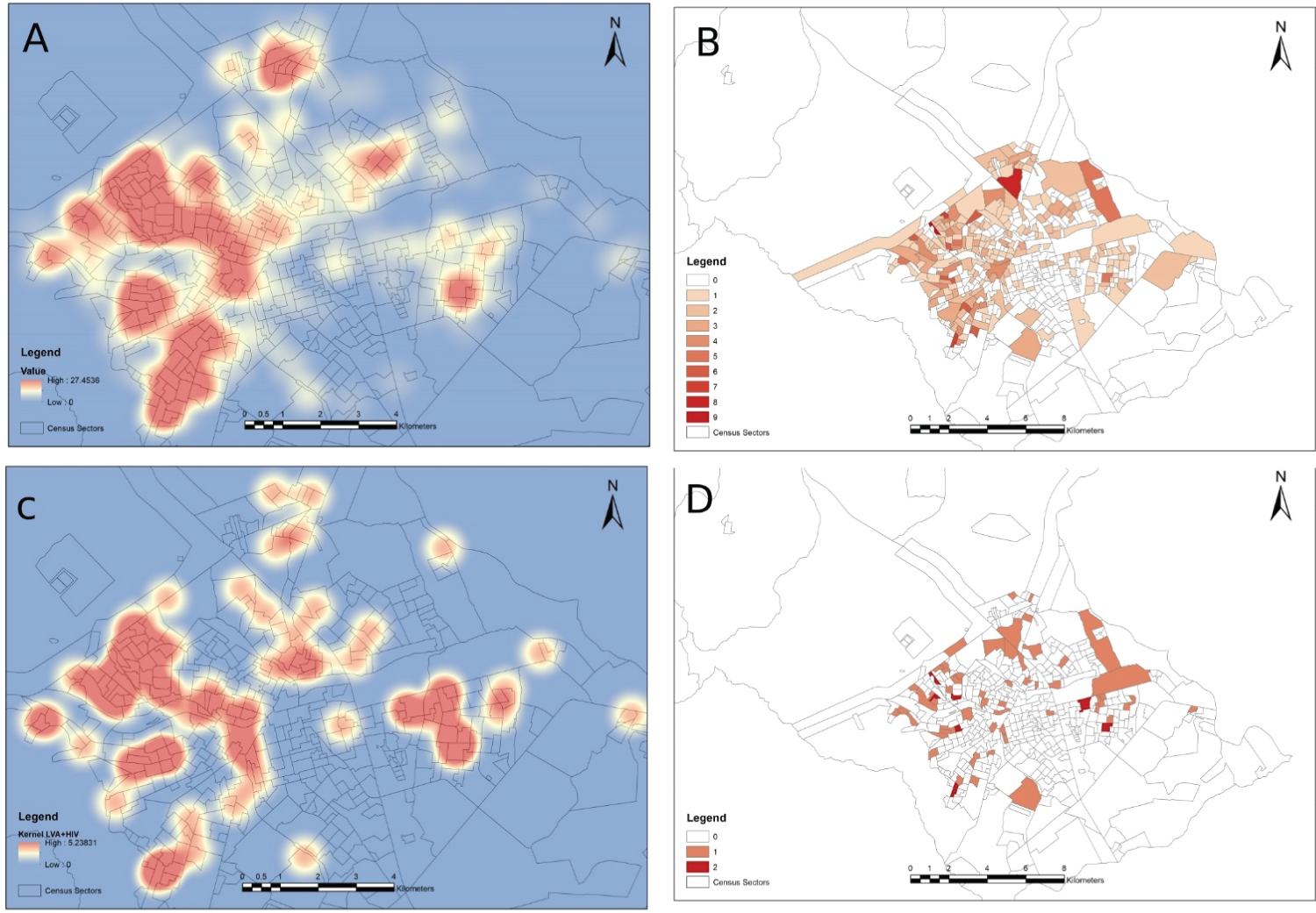 Figure 1: Spatial distribution of Visceral Leishmaniasis (VL) and VL-HIV coinfection in the city of Bauru, inner Brazil (A,B) Kernel density map and absolute incidence of overall VL; (C,D) Kernel density map and absolute incidence of VL-HIV coinfection.
View Figure 1
Figure 1: Spatial distribution of Visceral Leishmaniasis (VL) and VL-HIV coinfection in the city of Bauru, inner Brazil (A,B) Kernel density map and absolute incidence of overall VL; (C,D) Kernel density map and absolute incidence of VL-HIV coinfection.
View Figure 1
Table 1: Factors associated with the incidence of Visceral Leishmaniasis (VL) and VL-HIV coinfection among census sectors in the city of Bauru, inner Brazil. View Table 1
Our results provide clues to the routes of urbanization of VL and intersection of VL and AIDS epidemics. In univariable analysis, several aspects linked to poverty (e.g., absence of sidewalks and paving of streets, presence of garbage in the open) were associated with the outcomes. Those environmental factors may be proxies for both the presence of vectors (Lutzomyia longipalpis) breeding sites (usually sites with decaying organic matter) and of reservoirs (domiciled or stray dogs) [7,8].
Similarly to our results, proxy indicators of poverty such as (illiteracy and income) have been described both in areas of early [9] and recent emergence [10]. Urban VL has been associated with living in the periphery of cities, which are sites where favelas and poor neighborhoods are located. Not surprisingly, those are areas of greater prevalence of HIV infection [10].
Taken together, all those findings reinforce the social and economic determination in the distribution of VL and VL-coinfection. Since a recent systematic review failed to identify effective measures to prevent VL in Latin America [11,12], interventions aimed at improving income and housing conditions may be pathways for controlling this disease.