Background: Asparagus africanus has been claimed to possess antidiabetic activity in the ethnomedicinal literature. The present study was carried out to investigate the effect of aqueous extract of A. africanus (EAA) in control of renal complications in experimentally-induced type 2 diabetes mellitus in Wistar rats.
Methods: Prior to the administration in rats, the acute toxicity effect of the plant was assessed in mice. Fat diet/streptozotocin -induced type 2 diabetic Wistar rats treated orally with the EAA. Body weight was measured at specific intermissions during a treatment of 28 days. At the end of the treatment period, Kidney weight and serum and urine creatinine, albumin and urea were investigated. It was also evaluated the renal redox biomarkers along with histopathological examination of the kidney.
Results: In diabetes rats, the aqueous extract of A. africanus significantly increased body weight. Additionally, aqueous extract of A. africanus also decreased the level of creatinine and blood urea nitrogen in serum with high level in urine. Moreover, aqueous extract of A. africanus significantly reduced the presence of albumin in urine. Furthermore, aqueous extract of A. africanus significantly increased the renal antioxidant biomarkers but reduced the oxidative inducing in low oxidative stress imbalance. The aqueous extract of A. africanus significantly reduced glomerulosclerosis and tubular atrophy.
Conclusion: Aqueous extract of A. africanus possesses a promised potential to slow the dysfunction in renal function, oxidative imbalance and tissue damage in diabetic rats.
Type 2 Diabetes, Nephropathy, A. africanus , Aqueous extract
Diabetes is a complex heterogeneous syndrome, with a world’s demography estimated to about 415 million adults in 2017 [1]. The continuous increase of prevalence of diabetes in both developed and developing countries challenged scientists to further conduct research in sourcing for potent therapeutic agents from natural sources for more efficient usage in the treatment and management of diabetes [2]. The disease is characterized by a high level glycemia. The over-production of ROS during diabetic status (hyperglycemia) constitutes the major causes of complications in diabetes including renal failure [3]. Diabetic nephropathy, associated with the end stage renal failure [4], has therefore attracted more than one strategy for the controls of diabetes [5]. Treatment of diabetes essentially consists to the management of glycaemia or prevention of the complications notably the progressive diabetic nephropathy. Available drugs are mostly represented by antihypertensive agents, particularly inhibitors of the renin-angiotensin system (RAS), angiotensin-converting enzyme (ACE), angiotensin receptor antagonists, or their combination [6-10]. Although such treatments slow down the progression toward the end stage renal diseases (ESRD), they are not capable of preventing the onset of diabetic nephropathy [11]. Hence, alongside with the conventional therapy, many diabetic patients also used the complementary and alternative therapies which the demand have been intensified in the past few years [12] particularly, the use of antioxidant has been recently associated with reduced risk of ESRD in diabetes [13]. But, such therapeutics remains limited as not much research has been done. Following the antioxidant potential and safety of natural products along with functional foods, many researches have focused on medicinal plant or their bioactive constituents.
Asparagus africanus (Asparagaceae) is small spiny deciduous tree which is traditionally described to treat fever, malaria, rheumatism, and hyperglycemia. Survey of the genus Asparagus revealed the presence of variable classes of compounds such as flavones, flavanones, flavone glycoside, alkaloids, sterols, tannins, terpenoids, glycerols, carotenoids, saponins, carbohydrates, free amino and fatty acids [14,15]. In our preliminary studies, the qualitative phytochemical analysis has also permitted us to highlight the different groups of compounds including saponins, alkaloids, tannins, flavonoids, phenols and sterols in the aqueous extract of A. africanus . Some of these phytoelements were commonly reported in control of oxidative stress and other pathology associated with the complications of diabetes. The present study highlighted some of the therapeutic potential of the aqueous extract of A. africanus in Type 2 diabetes by focusing on the effect against renal complications.
Sample of the plant, A. africanus was harvested from Doukoula Kharhay in the Far North region of Cameroon in July 2022. A specimen of the plant was taken to National herbarium and authenticated in comparison with the material collected by Seville 277 (No: 14443 SRFCam). To prepare the extract, samples of the plant were collected and dried using an oven at 40 °C. Dried plant was ground to powder using a blender. The aqueous extract was prepared by putting the fine powder in hot water (90 °C) for six (6) hours, after which it is was filtered using the Whatman 1 filter paper. The filtrate was then collected and dried at 40 °C in the oven. The dried matter, which constituted the extract, was collected and kept at 4 °C.
Adult albino rats (Wistar strain) of 3-months-old with an average weight of 250 g were used for this research. The animals were housed in cages under conditions of normal room temperature and 12 hours cycle of light and darkness. They have free access to standard rat pellet diet and tap water ad libitum. All animals were acclimatized to the working environment for 1 week before the beginning of the experiment. Also, we had adult male (3-months-old) mice which were used for toxicity testing. The experimental protocol was approved by the Scientific Committee of the Department of Biochemistry.
Acute toxicity of the extract was evaluated using mice. Three different groups of 5 animals were made. Group 1 being the control group received distilled water (4 mL/Kg). Group 2 received 500 mg/Kg of the extract and group 3 received 1000 mg/Kg of the plant extract. The extract and water were administered orally by gavage and animals were observed for behavioural changes and mortality for a space of 14 days (2 weeks). The observation was recorded.
Type 2 diabetes was induced using low dose of STZ (35 mg/kg, i.p, prepared in 0.1 M citrate buffer pH 4.5) after 8 weeks of dietary modification using high fat diet. A control group received equal volume of vehicle (Distilled water) in a volume of 4 mL/kg. Fasting blood glucose (FBG) was monitored on day 2 after injection of STZ to confirm induction of diabetes and then on the 9 th day to ensure a stable diabetic condition. To monitor the fasting blood glucose (FBG), blood was collected from the tail vein and glucose meter (ACCU-ANSWER) was used. Rats with blood glucose level above 14 mmol/L or 200 mg/dl were considered as diabetic [16,17] and were selected for further study by initiating the treatment.
Selected diabetic animals and some normal animals were randomly allocated to 6 different groups of 5 animals each. The normal group, represented by the normal non-diabetic group, a diabetic non-treated group of animals representing the negative control; a diabetic metformin-treated group was used as a positive control. On the basis of oral acute toxicity studies and also following the criteria not to test extracts at doses that are not liable to have practical utility, three doses of EAA were administered to 3 different groups: 250 mg/kg, 500 mg/kg, and 1000 mg/kg. The normal and negative groups were receiving distilled water, while the positive group was receiving metformin (200 mg/kg in distilled water) [17]. Extract doses were prepared in distilled water and administered with the help of oral gavage once daily.
One day after ending the treatment, the rats were anesthetized with an intraperitoneal injection of ketamine and xylazine (50 mg/kg and 10 mg/kg, respectively) [18]. Using syringe, blood was used to withdraw from the tail veins of each animal and urine was collected directly from the bladder. Serum samples were obtained from blood after centrifugation at 3000 rpm for 10 mins. Kidneys were also collected, rinsed with isotonic saline and weighed using electronic balance. After weighing, the right kidney was kept in 10% formalin for histopathological evaluation. The left kidneys were minced and grind using a mortar and pestle, and were mixed with 10% (w/v) phosphate-buffer (0.1 M, pH 7.4). The homogenate was centrifuged at 4000 rpm for 10 minutes and the supernatant was collected and stored at 8 °C and used to estimate kidney antioxidant parameters.
Body weight: Measurements of body weight were recorded weekly during the treatment period. Body weight of rats was measured weekly using a mass balance and the weight gain was calculated.
Relative weight of kidney organ: Kidneys weight of rats was measured using a weighing balance. The recorded weight of two kidneys was used to estimate the relative kidney weight using the formula as follows [19]:
Serum and urine biochemical assays: Biochemical evaluation of renal function was carried out by estimating the serum and urine albumin, urea and creatinine levels using the spectrophotometric reagents commercially available kits. Creatinine concentration in serum was measured using the Creatinine K ® commercial kits (SGM ITALIA: Via Olivadi, 20 00126 Roma), which is based on the modified-Jaffe reaction. For the purpose, 100 µL of the serum sample was added to 1000 µL of alkaline picrate (working reagent) mixed for 30 seconds in water bath at 37 °C and absorbance read (E1C) at 510 and second absorbance (E2C) read after 2 mins. For the blank, 100 µL of distilled water was used in place of serum sample and the commercial standard solution 100 µL used in the place of sample for standard.
The analysis of urea was done using the commercial kit (SGM ITALIA: Via Olivadi, 20 00126 Roma). In details, 10 µL of serum sample and 10 µL of standard were added to different test-tubes (samples and standard tubes) containing 1000 µL of the working regent and to another tube was added 10 µL of distilled water (blank). The tubes were incubated in a water bath for 30 seconds at 37 °C after which absorbance read at 340 nm E1C and E1STD for sample and standard respectively and second reading was done after 2 minutes (E2C and E2STD for sample and standard respectively) and results recorded. Urea concentration in mg/dL was calculated as follows:
Blood urea nitrogen (BUN) was estimated as follows:
BUN (mg/dL) = Urea (mg/dL) × 0.467
For albumin, the analysis was done using the commercial kit (CHRONOLABS SYETEM S.L C/Diputacion 260, 08007, Barcelona, Spain). Briefly, 25 µL of serum or urine sample and 25 µL of a standard and 25 µL of distilled water were placed in their respective test tubes. To each tube 1000 µL of reagent was added. The tubes were incubated at 35 °C for 5 mins and the absorbance was then read at 540 nm.
This test is based on iron catalysed breakdown of hydroperoxides into alkoxyl (RO) and peroxyl (ROO) radicals which reacts with the chromogen (N, N-dimethyl-phenylenediamine sulphate) towards formation of a coloured compound and absorbance read at 505 nm [18]. According to the Lambert-Beer’s law, the intensity of the colour is directly correlated with the quantity of radical compounds, and can be reported as the oxidative status of the sample. In details, 100 µL of kidney homogenate diluted 10 times in phosphate buffer saline (PBS), was dissolved in 1 mL of acetate buffer. 25 µL of working chromogen solution was added, and absorbance read at 505 nm using a spectrophotometer against the blank.
In an acidic medium (pH = 5.2) and a suitable oxidant (FeCl 3 ), the chromogen (N, N-dimethyl-phenylenediamine sulphate) develops a stable and coloured radical cation that is photometrically detectable at 505 nm [18]. Antioxidant compounds in the sample reduce the radical cation of the chromogen, quenching the colour and producing a discoloration of the solution, which is proportional to their concentration. In practice, 1 mL of acetate buffer (pH = 5.2) was placed in test tube, and 25 µL of chromogen, followed by 10 µL of FeCl 3 . 10 µL of kidney homogenate diluted 20 times in PBS was added in the test tubes, and absorbance read at 505 nm using a spectrophotometer against the blank.
Oxidative stress and redox imbalance (OSI) of each sample was calculated as the ratio of TOS and TAS as follows: OSI = TOS [µmol/L]/TAS [µmol/L] × 100. Higher ratios in the samples are a sign of predominance of oxidation processes over antioxidant activity [20].
Nitric oxide concentration of each sample was determined by measuring the oxidative product of NO, the nitrite, using the Griess reaction [21]. Dithio-2,2-dithio-5,5'-dibenzoic acid (DTNB), a compound that reacts with the SH groups of the glutathione to form a yellow coloured complex that can be absorbed at 412 nm was used to measure the glutathione. Lipid Peroxidation was used to determine the MDA level and the TBARS test as reported by Wilbur, et al . [22] has been used. The ability of SOD to inhibit auto-oxidation of adrenaline to adrenochrome at pH 10.2 was used to evaluate its activities following the methods reported by Alex, et al. [23]. The method based on the reduction of dichromate in acetic acid to chromic acetate when heated in the presence of H 2 O 2 with the formation of per chloric acid was used to determine the level of catalase [24].
The kidney was collected and kept in formol 10% until the day of histological analysis. The histological analysis was done as follows: the distal portion of the kidney was cut and fixed with 10% neutral buffered formalin and then dehydrated with grades of ethanol (70, 80, 90, 95, and 100%). Sample was dehydrated and cleared in 2 changes of xylene. Samples were embedded and blocked out after impregnating with 2 changes of molten paraffin wax. Sections of 4-6 µm thickness cut using a microtome, mounted on glass slides and stained with hematoxylin and eosin (H and E). Stained sections of control and treated rats were examined using a panoramic viewer (3DHISTECH, Budapest, Hungary).
Data obtained from the experiments are expressed as mean ± standard error of mean (SEM). For statistical analysis, data were subjected to one-way analysis of variance (ANOVA) followed by Student’s t-test. A level of P < 0.05 was taken as significant. SPSS for windows was used for these statistical analyses.
Oral administration of extract (500 and 1000 mg/Kg) to determine the non-lethal dose for treatment, did not exhibit any signs of toxicity in terms of mortality during the observational period of 14 days. No changes in terms of feeding, movement or any other observational behavioural activity was observed and the aqueous extract of A. africanus is not lethal at the dose of 1000 mg/Kg (Table 1).
Table 1: Mortality and behavioural characters of animals in acute toxicity. View Table 1
The comparison of the weekly body weight gain of the animals showed that the treatment with the extract and metformin maintained invariable the weight gain during the treatment period while a decrease was observed in the negative group. At the end of the experimentation, the relative body weight of the rats receiving the extract showed no significant (p > 0.05) difference to those receiving metformin. Although, the body weights of the animals receiving EAA (500 mg/Kg, 1000 mg/Kg) were significantly higher than that of the negative control (p = 0.02, p = 0.046, respectively). Similarly, the weight of the rats receiving metformin, showed a significantly higher (p = 0.02) weight compared to the negative control (Figure 1).
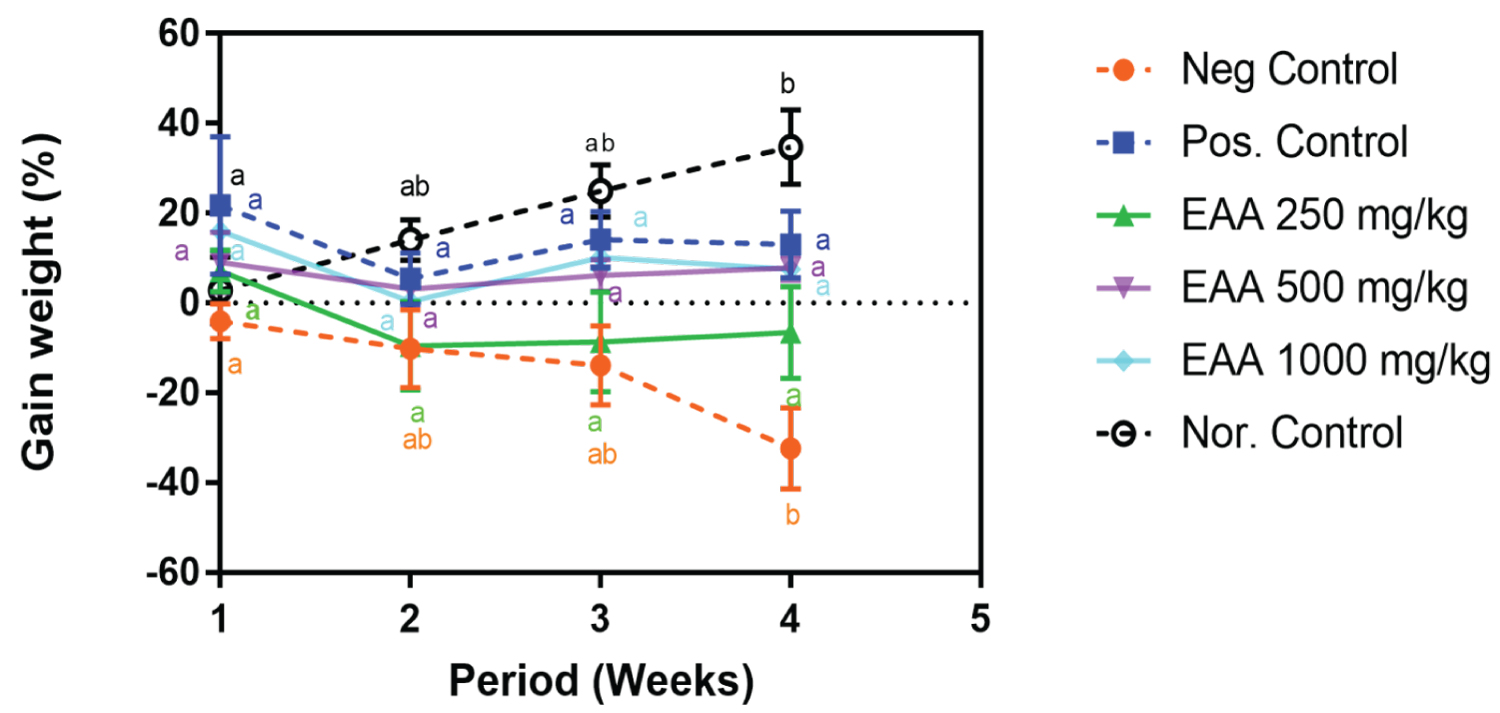 Figure 1: Weekly relative body weight (%) following treatment with A. africanus aqueous extract and metformin in STZ-induced diabetic rats. (Key: The letters indicates the difference between the body weights at different week for the same treatment. Same letters indicate insignificant difference).
View Figure 1
Figure 1: Weekly relative body weight (%) following treatment with A. africanus aqueous extract and metformin in STZ-induced diabetic rats. (Key: The letters indicates the difference between the body weights at different week for the same treatment. Same letters indicate insignificant difference).
View Figure 1
Results showed that the kidney weight was high in the non-treated diabetic group (1.84 ± 0.10). A significant difference was observed between the average kidney weight of the negative control and animals receiving extract at dose 1000 mg/Kg (p = 0.03). No significant difference (p > 0.05) was seen between all the treated groups for both receiving the extract and metformin. This phenomenon was also observed between the treated animals and the non-diabetic rats. In addition, results showed that there was a relative increase in kidney to body weight for the untreated diabetic animals (0.98 ± 0.19) and this was significantly (p < 0.05) ameliorated by treatment with both extracts (in a dose dependent manner) and metformin. Furthermore, there was no significant difference (p > 0.05) between relative kidney with groups receiving the extract, those receiving metformin and the non-diabetic rats (Table 2).
Table 2: Kidney weight (g) with respect to different treatment with A. africanus aqueous extract (EAA) and metformin in diabetic rats. View Table 2
As presented in Table 3, the results showed a marked decreased of urea and increased in BUN levels in urine of non-treated diabetic rats compared to that of the normal rats. EAA similarly to metformin significantly restored urine urea and BUN levels of diabetic rat back to normal. In addition, the results showed a significant decrease in the level of urine creatinine and significant increase in serum creatinine level compared to that of the normal rats. the effect of EAA on serum and urine was significant at doses with high level seen at dose 1000 mg/kg. The effect of EAA was similar to that of metformin (p > 0.05). Moreover, the results showed that the non-treated diabetic rats (Negative control) have a significant low serum albumin and high level of urine albumin compared to the normal rat (Nor. Control), EAA-treated and positive control.
Table 3: Serum and urine urea, creatinine and albumin concentrations following the different treatment with A. africanus aqueous extract (EAA) and metformin in diabetic rats. View Table 3
The Table 4 presents the effect of the EAA on the oxidative stress biomarkers. The results showed that TOS level is significantly low and TAD is high in group treated with EAA with maximum effect seen at dose 1000 mg/Kg compared to the negative control. The OSI calculated was lower in treated groups than in negative control. Results also showed that the animals receiving the extract (500 mg/Kg and 1000 mg/Kg) or metformin has a significant low MDA compared to negative control. Moreover, the result showed that the negative group showed a high level of NO compared to normal group and diabetic treated with EAA and metformin Furthermore, the results indicated that EAA-treated and positive control have significant level of GSH, SOD and CAT in a dose dependent manner compared to the negative control.
Table 4: Renal Redox biomarkers following the different treatment with A. africanus aqueous extract (EAA) and metformin in diabetic rats. View Table 4
Figure 2 showed the histopathological investigations of kidney indicating the glomerulus, Bowman’s space and the tubule. The cortex of kidney of rat from the negative control showed a glomerulus with severe nodules in diabetic glomerulosclerosis, wide bowman’s space, and tubular atrophy (Figure 2A) compared to the section from the cortex of kidney in a normal rat that showed a normal glomerulus, Bowman’s space, and normal proximal tubules (Figure 2B). In diabetic animals receiving the extract, the section from the cortex of kidney from rat taken 250 mg/kg showed a glomerulus with severe taking nodules in diabetic glomerulosclerosis, width bowman’s space, and tubular atrophy (Figure 2D) similarly to the negative control, the section from the cortex of kidney of rat taking extract at 500 mg/kg showed a glomerulus with diffuse glomerulosclerosis, a reduced bowman’s space compared to the negative control and proximal tubules similar to that of normal rat (Figure 2E). The section from the cortex of kidney from rat taken the extract at 1000 mg/kg, showed a normal glomerulus, bowman’s space, and proximal tubules similar to that normal rats (Figure 2F). Furthermore, the section from the cortex of kidney animals from positive control showed a glomerulus with diffuse glomerulosclerosis, Bowman’s space, normal proximal tubules and tubular atrophy (Figure 2C).
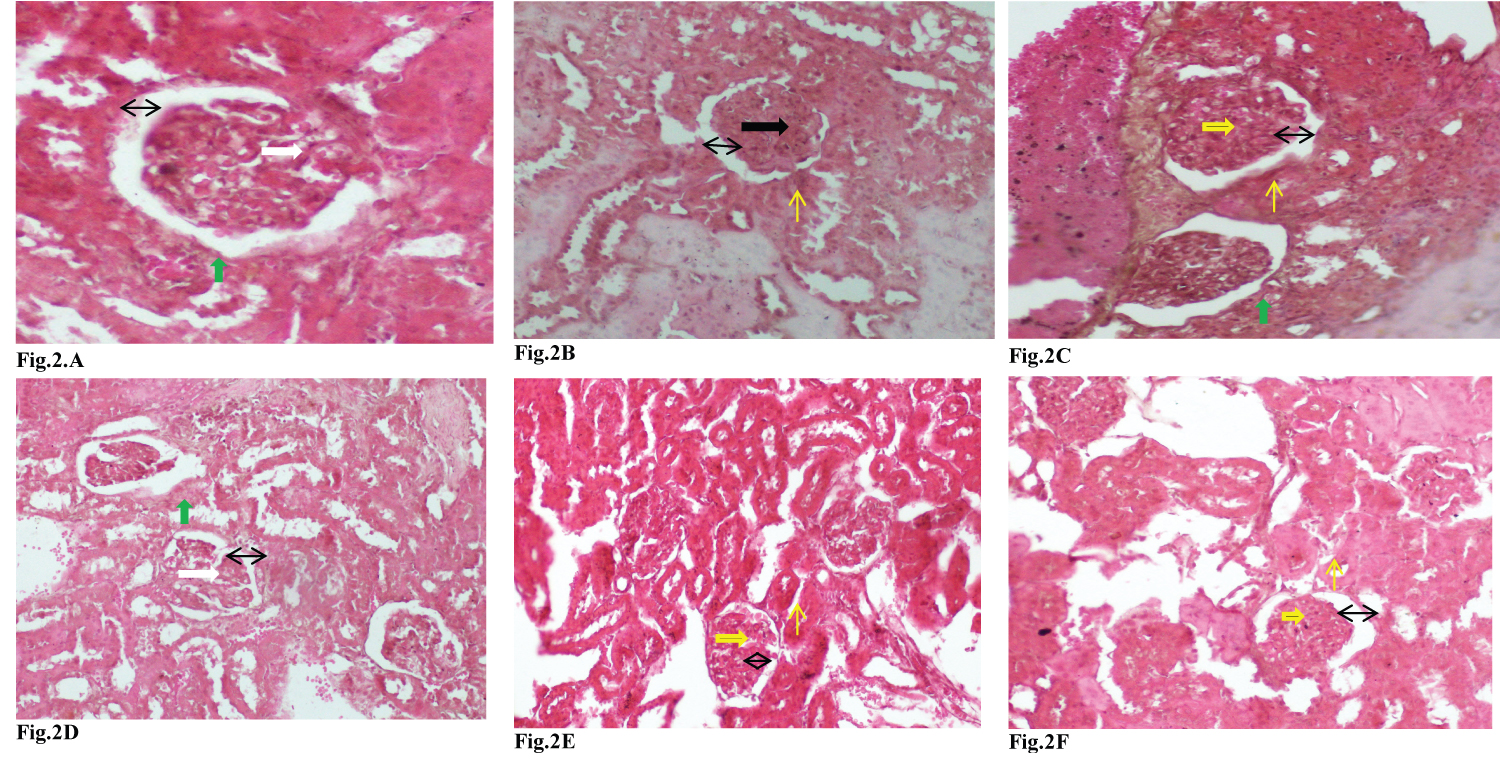 Figure 2: Photomicrograph of 3 microns thick H&E stained paraffin section from the cortex of kidney in rat following the treatments showing the glomerulus, tubule and the bowman’s space x 100. (Key: A glomerulus with diffuse glomerulosclerosis (→), a normal glomerulus (→), a glomerulus with severe nodules in diabetic glomerulosclerosis (→), Bowman’s space (↔), normal proximal tubules (↑) and tubular atrophy (↑)).
View Figure 2
Figure 2: Photomicrograph of 3 microns thick H&E stained paraffin section from the cortex of kidney in rat following the treatments showing the glomerulus, tubule and the bowman’s space x 100. (Key: A glomerulus with diffuse glomerulosclerosis (→), a normal glomerulus (→), a glomerulus with severe nodules in diabetic glomerulosclerosis (→), Bowman’s space (↔), normal proximal tubules (↑) and tubular atrophy (↑)).
View Figure 2
Herbal extracts with hypoglycaemic properties have been cumulatively reported to be having reverse effects on some physiological processes associated with deterioration in renal function, as well as in diabetes [5]. This study was focused on assessing the renoprotective effect of A. africanus in diabetic Witar rats. In the acute toxicity study, EAA at 1000 mg/Kg did not causes any changes in terms of feeding, movement and behavior. Therefore EAA is not toxic. The present study showed that the body weight of diabetic rats increased, and the treatement with EAA prevented this decreased in body weight seen in STZ-diabetic Wistar rats. STZ-induced diabetes is associated with significant reduction in the body weight due to hyperglycemia, increased muscle wasting and loss of tissue proteins [13,25]. This may indicate that the extract may prevent muscle tissue damage caused by hyperglycemia. The results of this study showed an increase in the weight of kidney (hypertrophy) in proportion to the body weight in STZ-induced rats. It was reported that local alterations in the production of growth factors such as the over expression of transforming growth factor-beta-1 in the kidney notably in proximal convoluted tubules cells and glomerular mesangial cells may result in the development of renal hypertrophy. An increase in the rate of protein synthesis and/or decrease in the degradation of renal extracellular components might also lead to renal hypertrophy [26]. EAA treatment reduced kidney/body weight ratio, thus demonstrating reversal of kidney hypertrophy in STZ-diabetic rats.
Because of increase and perfusion of concentration of residual products formed as a result of metabolism in kidney tubules, the kidneys are easily injured organs [27]. The levels of urea and creatinine in serum are used as indicators for determination of kidney functions; however, concentration of serum creatinine is a more effective indicator than urea concentration at the initial stages of kidney diseases [28]. Abnormal results in creatinine and blood urine nitrogen (BUN) are generally first indicators of kidney diseases [29]. From the study we observed an increase in serum creatinine and BUN in diabetic non treated rats, which is indicative of the resultant nephropathic diabetes. This effect was significantly regulated on treatment with extract, which can be explained by the extracts ability to regulate blood glucose levels, oxidative stress and regulation of damage to kidney caused by diabetes.
The body naturally produces reactive oxygen species which serve to destroy pathogens, but uncontrolled production leads to the oxidization of self-lipids, proteins, and DNA, thereby stimulating apoptosis [30]. Lipid peroxidation is one of the characteristic features of chronic diabetes leading to diabetic nephropathy. Malonaldehyde (MDA) is a product of the final stage of lipid peroxidation and an increase in hepatic and renal MDA is an index of enhanced lipid peroxidation in diabetes [13]. From the result we obtained, the concentration of MDA was seen to be higher in the non-treated diabetic animals (negative control) while the concentration was seen to significantly decrease in a dose dependent manner in animals receiving EAA, hence the extract had the ability to alleviate lipid peroxidation which can be due to its antioxidant potentials. Hyperglycemia leads to increased production of reactive oxygen species (ROS) which are involved in the etiology of several diabetic complications including diabetic nephropathy [25]. The reactive oxygen species deplete the antioxidant defense of the cell thus making it more susceptible to oxidative damage. The quantity of the H 2 0 2 was seen to significantly decrease in the treated groups while increased HP levels in kidney was observed in diabetic rats as opposed to its reduction in groups receiving treatments. It may be due to increased activity of antioxidant enzymes and the increase in concentration total antioxidant species. This therefor indicates the potential of the extract to scavenge the H 2 O 2 species and increase antioxidant quantities in the kidney.
In diabetes, vascular complications were highly associated with the oxidative status [31], which may be due to ROS level elevation, a result of destruction or/and decrease in the production by catalase (CAT-enzymatic/non-enzymatic), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) antioxidants. Variation in the concentration of one or more of these enzymes in an organ make the tissues susceptible to oxidative stress leading to the development of diabetic complications [32]. Chronic hyperglycemia is often associated with a significant decline in intracellular antioxidants and an elevation in the formation of pro-oxidants such as reactive free radicals and electrophilic substances eventually resulting in renal dysfunction and deterioration [26]. Superoxide dismutase provides first line defense against ROS mediated cell injury by catalysing the proportion of superoxide, the primary ROS in oxygen metabolism, to molecular oxygen and water. Superoxide is therefore dissimulated to other compounds that are less toxic by SODs [33]. Scientists claimed that, the treatment of diabetes with antioxidant therapy is like applying water to a burning house and is certainly helpful in limiting damage. The EAA showed a significant increase in SOD levels in diabetic rats. This therefore suggests its ability to reverse oxidative stress by increasing the quantities of SOD in tissues and overcoming the on-slot of diabetic complication. CAT converts H 2 O 2 catalytically into water and oxygen and thus neutralizes it, which in case of catalase deficiency, beta cell of pancreas that contain large amount of mitochondria, undergoes oxidative stress by producing excess ROS that leads to b-cells dysfunction and ultimately diabetes [34]. In the study the EAA showed a very high significant increase in the concentration of catalase, much more than SOD and GSH. This reveals the ability of the extract to promote the production of the enzyme which is very crucial in reversing or preventing oxidative stress. Diabetes induces alterations in activity of enzymes glutathione peroxidase and glutathione reductase which are found in cell that metabolizes peroxide to water and converting glutathione disulfide back into glutathione. Depletion of kidney GSH levels represents enhanced oxidative stress [34]. The results showed that the extract significantly increase the concentration of the GSH antioxidant which further shows the effectiveness of the extract of Asparagus africanus against oxidative stress in the kidney and its further illustrates its ability to prevent or limit diabetic complications.
The histopathological examination result in this study agrees with Zafar, et al. [35] and Alipin, et al. [36] who found that the relative kidney weight in STZ-induced rat for 12 weeks was significantly higher than that of the non-induced rat. In this study, the Bowman's space in kidneys of diabetic rats was wider than of non-diabetic rats. Bowman's space widening is caused by the expansion of the Bowman capsule when glucose enters kidneys [37], which also associated with increased absorption and accumulation of glycogen in kidneys [38] which caused nephropathy in diabetic disease model. Treatment with extract showed improvement in kidney histological structure as indicated by decreased of glomerular diameter, narrowed of Bowman space, and decreased of the necrotized proximal tubules at dose 1000 mg/Kg.
The present investigation showed that the aqueous extract of A. africanus possesses a promised potential to slow dysfunction in renal function, oxidative balance and tissue damage in diabetic rats.
The experimental protocol was approved by the Departmental Scientific of the Department of Biochemistry and animals were handled by respecting the protocol of the Cameroon National Veterinary Laboratory (003/19 CCS/MINEPIA/RD-NW/DDME/SSV).
Not applicable.
All authors read and approved the publication the manuscript.
All relevant data are within the paper and its supporting information files.
The authors have declared that no competing interest exists.
Not applicable.
FEN: Collection, interpretation of data and manuscript drafting. BBM: Revised the paper. OM and TC: Conception of the research idea, designing, data analysis and manuscript drafting. All authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Authors are grateful to Prof. Germain Taiwe Sotoing, University of Buea, Cameroon, for supporting our this specific project by facilatating the purchase of reagent.