Comparison of Bioelectrical Impedance Analysis and Magnetic Resonance Imaging for the Quantification of Fat Mass
Cuisle Forde1*, Niamh Murphy1, James Meaney2, Paul Kennedy3, Gerard Boyle3 and John Gormley1
1Discipline of Physiotherapy, Trinity College Dublin, Ireland
2Department of Radiology, St. James's Hospital, Ireland
3Department of Medical Physics & Bioengineering, St. James's Hospital, Ireland
*Corresponding author:
Cuisle Forde, Discipline of Physiotherapy, Trinity College Dublin, Ireland, Tel: +35318962128, E-mail: c.forde@tcd.ie
Int J Physiatry,
ijp-1-003, (Volume 1, Issue 1),
Original Research Article
Received: July 22, 2015: Accepted: August 13, 2015: Published: August 17, 2015
Citation:Forde C, Murphy N, Meaney J, Kennedy P, Boyle G, et al. (2015) Comparison of Bioelectrical Impedance Analysis and Magnetic Resonance Imaging for
the Quantification of Fat Mass. Int J Physiatry 1:003
Copyright: © 2015 Murphy N. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution,and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Body composition is a key determinant of health. Many methods, including bioelectrical impedance analysis (BIA), are available to estimate body composition; however the validity of such methods varies greatly. The aim of this cross sectional study was to investigate the accuracy of the Tanita MC-180MA BIA machine in the quantification of fat mass through comparison with magnetic resonance imaging (MRI).
Methods: Ten young healthy volunteers (aged 24.4, SD 3.8 years) underwent BIA and MRI. Fat mass quantification was generated by the system software of the MC-180MA through the use of prediction equations and impedance data, and through the use of a bespoke application developed for use with MRI output. Fat mass results for the lower limbs and torso were analysed using correlation, t-test and Bland and Altman analysis.
Results: Strong correlation was observed however there was a significant difference between assessment methods for fat mass in the lower limb and in the lower limbs and torso combined (p=0.002 and p=0.013 respectively). Bland and Altman plots showed large limits of agreement spanning up to 54% of the overall mean and significant bias for measurement of fat mass in the lower limbs (2.2kg) and torso and lower limbs combined (2.6kg).
Conclusions: When compared to results obtained during MRI, BIA significantly underestimated fat mass. Overall, accuracy was poor and it can be concluded that BIA showed poor agreement with MRI in the quantification of fat mass.
Keywords
Fat, Adiposity, Torso, Assessment
Introduction
Increased fat mass is related to the metabolic syndrome [1], cardiovascular disease [2], type two diabetes [3], and certain types of cancer [4,5] as well as increased mortality and morbidity [6]. Diseases associated with a high fat mass have also been shown to be present among those with increased body fat who have a normal BMI [2,7,8]. There is therefore an interest in the estimation of body composition, particularly body fat, in clinical settings.
There are many different methods used to estimate body composition. These techniques range from being simple and accessible, such as body mass index (BMI) to complex and expensive such as isotope dilution. All techniques used to estimate body composition can be broadly categorised into two groups, those suitable for routine clinical use, and those not suitable for routine clinical use. Common clinical tools used to estimate body composition such as skin fold thickness, BMI and waist hip ratio are useful, however their accuracy and repeatability have been shown to be low, particularly in untrained assessors [9], and when self reported [10].
Bioelectrical impedance analysis (BIA) is a cost effective, quick and safe method of assessing body composition. It has high repeatability [11] and unlike BMI and waist hip ratio, can provide information on body fat distribution. These advantages over other methods of assessing body composition have resulted in the routine use of BIA in clinical and research settings. The Tanita MC-180MA is an example of an eight-electrode multi frequency BIA machine that allows body fat mass to be estimated for each arm, each leg and the trunk.
Magnetic resonance imaging (MRI) can be used to measure body composition and has been shown to be a valid, accurate method of fat quantification in animals [12] and humans [13] when compared with dissection. A number of imaging approaches can be taken to visualise fat in MRI. For example in T1-weighted MRI imaging, tissues with short T1 appear brightest or 'hyperintense'. Fat has a very short T1 time and will appear as the brightest tissue in the image. In Dixon MRI imaging techniques separate fat and water images are generated, by exploiting differences in the precession frequency of fat and water in MRI. Whole body imaging is available on current MRI scanners, allowing these imaging techniques to generate high-resolution, multi-slice, head to toe images. However, whole body fat quantification is not yet a routinely available option and image processing steps need to be implemented to generate quantitative measures of body composition from the MRI images [14].
The validity of the Tanita MC-180MA in the estimation of body fat has previously been examined through comparison with dual X-ray absorptiometry (DXA) [15,16]. Results of these studies are inconsistent, reporting both underestimation and overestimation of body fat [15,16]. DXA is a valid measurement tool and is commonly used as a reference method, however concerns have been raised about the accuracy of using DXA to measure body fat in areas of high bone mass such as the thorax [17,18], for which MRI is believed to be a more optimal tool [19].
Browning et al. (2011) examined the validity of the MC-180MA through comparison with MRI [20], however only the abdominal area was included. Although the MC-180MA output includes a "visceral fat level" reading, the machine is not designed for the quantification of abdominal or visceral fat alone, but rather total thoracic fat mass. No study to date has compared quantification of fat mass between the MC-180MA and MRI. As BIA is used in a lot of clinical and research settings for the quantification of fat mass, there is a clear need to validate such measurements through comparison with MRI. This is the first study to investigate the accuracy of the Multi-Frequency Body Composition Analyser MC-180MA at quantifying fat mass through comparison with MRI.
Material and Methods
Recruitment
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Joint Research Ethics Committee of the Adelaide and Meath Hospital incorporating the National Children's Hospital and St. James's Hospital. Written informed consent and an MRI contraindications form were completed by all subjects and checked by the primary investigator and radiographer prior to any measurements being taken. Inclusion criteria were the ability to stand unaided and the ability to perform all pre-test instructions. Pre-test instructions as per the MC-180MA manufacturer guidelines were to fast for three hours prior to testing; refrain from excessive fluid intake for 12 hours prior to testing (i.e. not to drink more than usual); avoid strenuous exercise for 12 hours prior to testing; avoid alcoholic beverage consumption for 12 hours prior to testing; and void the bladder within 30 minutes prior to testing. These instructions were necessary to ensure participants were adequately but not over-hydrated since hydration status can affect bioelectrical impedance measurements [21]. Participants were excluded if they had any metal in their body, were pregnant or suffered from claustrophobia.
Measurements
This study was cross sectional in nature. Body height was measured to the nearest 0.2cm using a stadiometer (Seca Mod 220, Hamburg, Germany). Body weight (measured to the nearest 0.05kg) and fat mass were estimated using the Multi-Frequency Body Composition Analyser MC-180MA. Fat mass was also estimated using whole body MRI (3T Philis Achieva MRI Scanner, Philips Amsterdam, and The Netherlands). Participants were scanned in the supine position using an eight station whole body imaging protocol to achieve full body coverage. The sequence used was a T1-weighted, TFE sequence with water suppression (echo time=2.3 ms, repetition time=774 MS, WFS=1 pixel, flip angle 70 degrees, spectral presaturation inversion recovery (SPIR) water suppression, NSA=2, integrated body coil). This weighting ensures that body fat is shown as hyperintense in relation to other structures in the image. The resolution of multi-slice 2D images in the coronal place was 1.83mm by 2.48mm with a slice gap of 1mm. Coronal images were reconstructed on a 1.345mm by 1 .345mm interpolated matrix. Multiple breath- holding was used to reduce motion artefact while scanning the station over the lungs and upper abdomen. Each scan generated 30 whole body coronal images. Each coronal image consisted of eight images from each of the scanned stations fused together to form a single image. Fusing was achieved using the MRI's own image fusing software (MobiView™). Images were exported to a bespoke application written in MATLAB (Mathworks, Cambridge, UK) for fat quantification. Images were thresholded using a technique called Otsu's method [22]. At each station level image in a coronal slice, a threshold pixel grey level value was chosen above which a pixel is considered to represent a fat voxel. In Otsu's method the threshold is automatically chosen by minimising the grey level variance within the above and below threshold pixel populations. The resulting operator view is a stack of 30 coronal whole body 'binary' images with 'fat' showing white and all else black (Figure 1). These binary images could be further manually edited by the operator to cut out remaining bright pixels associated with bone marrow. The total fat volume with a region of interest was calculated by a simple count of the total number of voxels above threshold in that region.
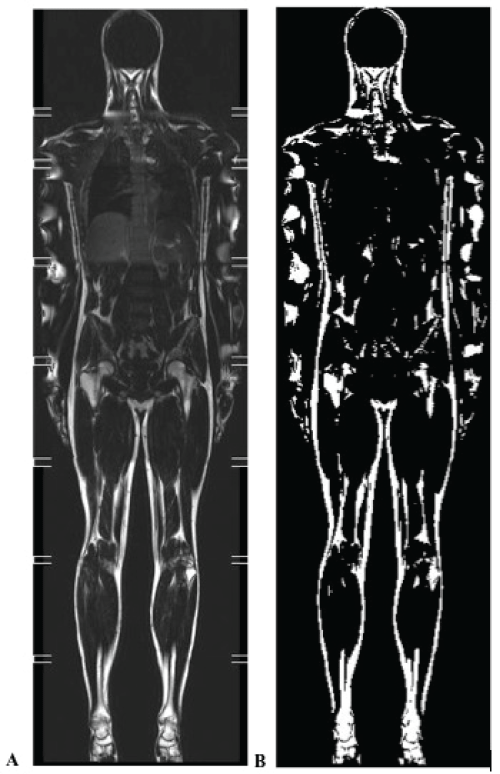
.
Figure 1: An example image showing an original whole body coronal image (A) and the corresponding thresholded 'fat' image (B). Note artefact on arms in A, causing water based tissues to be incorrectly included in B. Also note inclusion of bone marrow in B. Image B is further manually edited to limited analysis to a region of interest and to exclude marrow prior to fat quantification.
View Figure 1
In this study comparisons were made between fat mass estimates of the torso and lower limbs obtained by the MC-180MA and those obtained by MRI. Fat mass of the arms was not analysed since they were close to the edge of the MRI image bore, which leads to poor image quality. Due to their position a strong banding artefact can be present on the arms, which can lead to errors in the estimation of fat mass. The arms were therefore excluded from analysis. The torso was defined as from the upper level of the shoulders to the head of the femur. The lower limbs were defined as below the level of the head of the femur. The MRI output was fat volume per defined area (i.e. torso and lower limbs). Fat mass was subsequently estimated from fat volume by using a fat density estimate of 0.9g/cm3. MRI results were analysed by a trained medical physicist, who was blinded to the results from the bioelectrical impedance device.
Statistical analysis
Results were analysed using SPSS. Alpha of 0.05 was used to denote statistical significance. The Kolmogorov-Smirnov test was used to test for normality. Pearson product moment correlation coefficients, or Spearman's rho were calculated, where appropriate, to determine the association between measurements acquired with BIA and MRI where appropriate. Paired t tests or Wilcoxin tests were used to examine differences between results obtained with BIA and MRI. Finally, Bland and Altman plots were produced to test the agreement between results obtained. In Bland and Altman analysis, the bias was described as the mean difference in results between the measurement methods. The bias was compared to zero.
Results
Ten participants (eight female) were recruited and completed this study. The sample size is similar to other studies using MRI in the investigation of body composition, and was considered feasible given budgetary constraints. Participant characteristics are presented in table1. Fat mass estimated using BIA and MRI, as well as comparisons between these estimations are detailed in table 2.
![]()
Table 1: Participant characteristics
View Table 1
![]()
Table 2: Fat mass quantification results
View Table 2
Table 2 shows strong correlations between MRI and BIA for fat mass in the torso, lower limbs and torso plus lower limbs. No significant difference was found between measurement methods for fat mass recorded for the torso. There were significant differences between measurement methods in fat mass results recorded for the lower limbs and torso plus lower limbs combined. Bland and Altman plots (Figures 2-4) show significant bias whereby BIA consistently underestimated fat mass in the lower limbs and lower limbs plus torso. Furthermore limits of agreement were wide for all measurement areas, spanning 44% of the overall mean for the torso, 38% for the lower limbs and 54% for the limbs and torso combined. For the purpose of result interpretation, a difference in fat mass between methods of greater than 2kg for measurements taken of the torso and of the lower limbs was considered clinically significant. Between 30% and 40% of subjects had results outside of this recommendation. For measurement of fat at the torso, three subjects had differences between methods of greater than 2kg (Figure 2). For the lower limbs this was increased to four subjects (Figure 3). For the torso and lower limbs combined three subjects had a difference between methods of greater than 4kg (Figure 4).
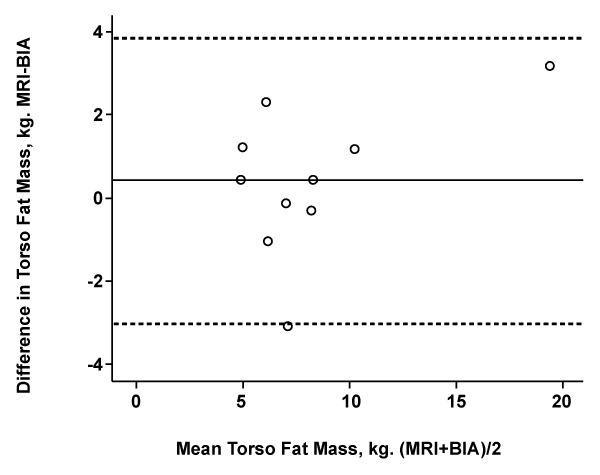
.
Figure 2: Bland and Altman plot of agreement between MRI and BIA for torso fat mass
Kg: Kilogram, MRI: Magnetic Resonance Imagining, BIA: Bioelectrical Impedance Analysis Machine.
View Figure 2
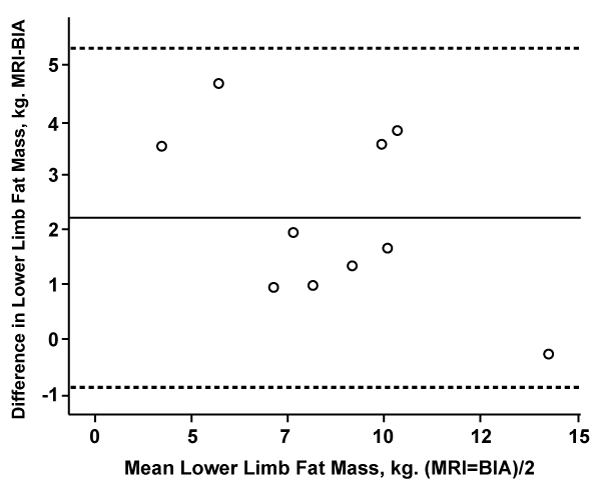
.
Figure 3: Bland and Altman plot of agreement between MRI and BIA for lower limb fat mass
Kg: Kilogram, MRI: Magnetic Resonance Imagining, BIA: Bioelectrical Impedance Analysis Machine
View Figure 3
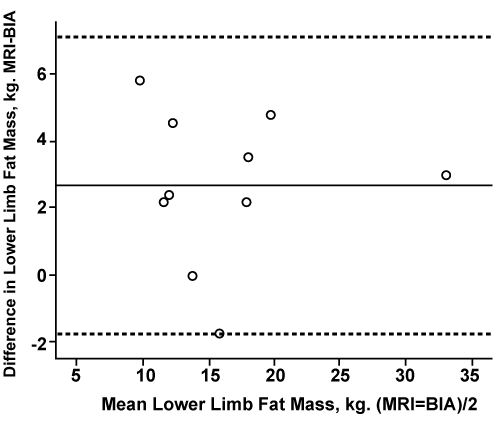
.
Figure 4: Bland and Altman plot of agreement between MRI and BIA for torso+lower limb fat mass
Kg: Kilogram, MRI: Magnetic Resonance Imagining, BIA: Bioelectrical Impedance Analysis Machine
View Figure 4
Discussion
Results of this study have shown that overall the MC-180MA has poor accuracy in the quantification of fat mass. Large limits of agreement were seen in Bland Altman analysis with significant bias indicating that the MC-180MA can result in significant underestimation of fat mass. In the current study, limits of agreement spanned 6.9kg for the torso, 6.2kg for the lower limbs and 8.9kg for the torso and lower limbs combined. Since the average fat mass results in the torso and lower limbs was 8.25kg and 8.17kg respectively, the limits of agreement are considered to be very large.
With increasing evidence that body composition and in particular fat distribution plays an important role in health, several methods have become available to facilitate quick and cost effective estimation of body fat distribution. The MC-180MA is one such tool; however there is limited evidence to support the validity of this machine in the quantification of fat mass. This study compared fat mass estimated by the MC-180MA with that estimated using MRI.
Most studies to date examining the validity of various BIA machines have employed DXA as a reference assessment method [15,16,23-28]. Results of many of these studies have shown a tendency for BIA to underestimate fat mass [15,16,24-27]. There have also been reports of BIA showing no significant bias compared to DXA [23], and in some cases an overestimation of fat [15,25].
Few studies have compared BIA to MRI. There is a need for more work to be carried out comparing these two methods since there are clear differences between MRI and DXA as reference methods. Of those that have used MRI to validate BIA, Varady et al. (2007) investigated whole body composition [29] while others only investigated abdominal adiposity [20,30-32]. Similar to results from studies using DXA as a reference method, Varady et al. (2007) found significant underestimations of body fat with BIA [29]. Studies examining BIA as a tool to estimate abdominal adiposity reported that it was useful as a proxy for total abdominal adipose tissue measured by MRI, but not for visceral adiposity [20,30,32]. It is worth noting that the MC-180MA was not designed to measure visceral fat and the separate quantification of visceral fat by MRI was not an aim of this study, therefore we cannot comment on the accuracy of the MC-180MA in this regard.
Specific to the Tanita MC-180MA, three previous studies have examined the validity of fat mass measurement through comparison with either DXA [15,16] or MRI [20]. Leahy et al. (2012) found a significant underestimation of fat tissue mass by the MC-180MA when compared to DXA [15]. Interestingly, the underestimation was not consistent across all body segments or between sexes, with a significant underestimation of trunk fat mass among women, but a significant overestimation of trunk fat mass among men. Overall limits of agreements spanned 71% of the overall mean for men and 45% for women with a bias of 0.3kg and 1.7kg respectively. Nigam et al. (2013) investigated the validity of the MC-180MA to estimate fat mass in an Asian population (using the Asian specific equations developed for the MC-180MA) through comparison with DXA [16]. Results of this study also revealed an underestimation of fat mass with even greater bias (-5.4kg) and wider limits of agreement (139% of the overall mean). Results of the current study revealed biases lower than those reported by Leahy et al. (2012) and higher than those reported by Nigam et al. (2013). Limits of agreement were narrower than those reported by Nigam et al. (2013) and those reported for men by Leahy et al. (2012), but similar to those reported for women by Leahy et al. (2012). Differences in results observed are likely due to the difference in reference methods used (i.e. DXA rather than MRI) and populations examined. However despite methodological differences, all three aforementioned studies and the current study investigating the validity of the MC-180MA reported a significant underestimation of fat mass.
In the only study validating results of the MC-180MA through comparison with MRI, Browning et al. (2011) compared BMI, waist circumference, DXA and BIA to MRI measurements of abdominal adiposity [20]. Results were similar to those obtained in the present study with correlations between methods for total thoracic adiposity being high at r=0.89. Browning did not employ Bland and Altman analysis, however with regression analysis they concluded that the MC-180MA would be a useful correlate and proxy measure for total abdominal adipose tissue [20]. It is worth noting that Browning et al. (2011) used imaging of the abdomen only and only one MRI slice was used for analysis [20].
In this study, the accuracy of BIA was greater for adiposity deposited in the torso (bias of 0.4kg) than the lower limbs (bias of 2.2kg). This result highlights the advantages of segmental fat mass analysis, whereby results may be more valid for one segment than another. It is possible that, because the torso better resembles a cylindrical shape than the limbs BIA is more valid for use as a tool to estimate fat mass in this region, which accounts for a large proportion of the conductive mass but has little impedance [33]. Similar comparisons between body segments were made by Leahy et al. (2012), whose results conflicted with those found here; the MC-180MA was less accurate in measuring body fat % in the trunk region than the arms or legs [15]. The difference in results seen between the current study and that conducted by Leahy et al. when comparing body segments is likely attributable to the reference methods used.
There are several possible reasons for the disparity between results obtained from the MC-180MA and MRI in this study. Firstly, those obtained from the BIA are dependent on prediction equations and assumptions on hydration status. Although pre-measurement instructions were adhered to in this study it was not possible to ensure all participants were at the same hydration level. There is also some evidence to suggest that BIA is more suited to the measurement of subcutaneous fat than visceral fat [31]. It is possible that the ratio of visceral fat to total thoracic fat differed between participants, which may have explained some of the inaccuracy in results. Finally, the small number of participants in this study could be seen as a limitation as Bland and Altman analysis is suited to larger numbers [34], however the cost associated with whole body MRI prohibited a larger sample size.
A limitation of this study is that the MRI fat quantification technique used was not validated against another standard. While fat was clearly visualised in the MRI images, potential sources of error in the estimates of fat mass generated here do exist. As with all MRI techniques, images are subject to artefact and noise that can impact on quantitative measures. The arms, head and neck were excluded from analysis as a large number of artefacts occurred in these areas.
The imaging technique used does not reject fat in the bone marrow and this component of fat was edited out of the images before fat quantification. As the editing process is manual, it has the potential to introduce error. Furthermore, at fat boundaries, a partial volume effect can occur, whereby an entire voxel would be counted as fat even if it only partially overlapped the true fat volume.
Previous studies investigating the validity of BIA as a measure of fat mass have found that bias increased as the fat mass of the individual being examined increased [15,35,36]. In the current study, only one participant was overweight or obese (as categorised using BMI). With the exception of this individual, fat mass values were in the healthy range. Therefore the inaccuracy of reported fat mass quantification in this study cannot be attributed to participants having unhealthy quantities of body fat. Furthermore, the degree of error found in this study cannot be generalised to a population with an unhealthy BMI.
Consistent bias between methods leads us to conclude that the ability to assess body composition with the MC-180MA on an individual basis is limited. The results of this study support the view of other literature in the area, that BIA is not accurate in the quantification of fat mass. Other possible uses for BIA include the estimation of fat free mass [28] and the monitoring of changes in body composition for an individual or large groups, [26,,37] for which the validity of BIA appears more promising.
In light of the results of this and previous studies, it may be prudent for clinicians to examine the validity of the exact BIA tool used, especially for the quantification of fat mass. While BIA can give many results related to body composition in a matter of seconds, results of our study show that fat quantification may be underestimated. Giving that fat mass is a key determinant of health this is a cause for concern. Although results were more encouraging for fat mass measurement in the torso segment, which is the arguably the most important site for fat quantification, limits of agreement were still wide and 30% of subjects had differences in results between measurement tools of greater than 2kg.
This study demonstrates that the MC-180MA has limited accuracy in the quantification of fat mass for individuals where MRI is used as the reference standard and that BIA cannot be used as a proxy measure for MRI. High correlation between methods indicates good relative agreement, however absolute agreement between the methods was poor with limits of agreement spanning up to 54% of the mean. Results of this study lead us to question the validity of the MC-180MA in its quantification of fat mass for individuals.
Ethical Statement
As per the methods section; this study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Joint Research Ethics Committee of the Adelaide and Meath Hospital incorporating the National Children's Hospital and St. James's Hospital. Written informed consent and an MRI contraindications form were completed by all subjects and checked by the primary investigator and radiographer prior to any measurements being taken.
References
-
Zhu S, Wang Z, Shen W, Heymsfield SB, Heshka S (2003) Percentage body fat ranges associated with metabolic syndrome risk: results based on the third National Health and Nutrition Examination Survey (1988-1994). Am J Clin Nutr 78: 228-235.
-
Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A,et al. (1990) Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Ahteriosclerosis 10: 497-511.
-
Han TS, Feskens EJ, Lean ME, Seidell JC (1998) Associations of body composition with type 2 diabetes mellitus. Diabet Med 15: 129-35.
-
Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, et al. (2002) Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control 13: 741-751.
-
MacInnis RJ, English DR (2006) Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control 17: 989-1003.
-
Flegal KM, Kit BK, Orpana H, Graubard BI (2013) Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 309: 71-82.
-
Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, et al. (2008) Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 32: 959-966.
-
Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, et al. (2011) Assessing adiposity: a scientific statement from the American Heart Association. Circulation 124: 1996-2019.
-
Kerr L, Wilkerson S, Bandy WB, Ishee J (1994) Reliability and Validity of Skinfold Measurements of Trained Versus Untrained Testers. Isokinetics and Exercise Science 4: 137-140.
-
Scribani M, Shelton J, Chapel D, Krupa N, Wyckoff L, et al. (2014) Comparison of bias resulting from two methods of self-reporting height and weight: a validation study. JRSM Open 5.
-
Loenneke JP, Barnes JT, Wilson JM, Lowery RP, Isaacs MN, et al. (2013) Reliability of field methods for estimating body fat. Clin Physiol Funct Imaging 33: 405-408.
-
Fowler PA, Fuller MF, Glasbey CA, Cameron GG, Foster MA (1992) Validation of the in vivo measurement of adipose tissue by magnetic resonance imaging of lean and obese pigs. Am J Clin Nutr 56: 7-13.
-
Abate N, Burns D, Peshock RM, Garg A, Grundy SM (1994) Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35:1490-1496.
-
Ludwig UA, Klausmann F, Baumann S, Honal M, Hovener JB, et al. (2014) Whole-body MRI-based fat quantification: a comparison to air displacement plethysmography. J Magn Reson Imaging 40: 1437-1444.
-
Leahy S, O'Neill C, Sohun R, Jakeman P (2012) A comparison of dual energy X-ray absorptiometry and bioelectrical impedance analysis to measure total and segmental body composition in healthy young adults. Eur J Appl Physiol 112: 589-595.
-
Nigam P, Misra A, Colles SL (2013) Comparison of DEXA-derived body fat measurement to two race-specific bioelectrical impedance equations in healthy Indians. Diabetes Metab Syndr 7: 72-77.
-
Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB (1993) Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a "gold standard". Am J Clin Nutr 58: 589-591.
-
Genton L, Hans D, Kyle UG, Pichard C (2002) Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition 18: 66-70.
-
van der Kooy K, Seidell JC (1993) Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 17: 187-196.
-
Browning LM, Mugridge O, Dixon AK, Aitken SW, Prentice AM, et al. (2011) Measuring abdominal adipose tissue: comparison of simpler methods with MRI. Obes Facts 4: 9-15.
-
Abu Khaled M, McCutcheon MJ, Reddy S, Pearman PL, Hunter GR, et al. (1988) Electrical impedance in assessing human body composition: the BIA method. Am J Clin Nutr 47: 789-792.
-
Otsu N (1979) A threshold selection method from grey-level histograms. IEEE Transactions in Systems, Man and Cybernetics 9: 62-66.
-
Karelis AD, Chamberland G, Aubertin-Leheudre M, Duval C (2013) Validation of a portable bioelectrical impedance analyzer for the assessment of body composition. Appl Physiol Nutr Metab 38: 27-32.
-
Wang L, Hui SS, Wong SH (2014) Validity of bioelectrical impedance measurement in predicting fat-free mass of chinese children and adolescents. Med Sci Monit 20: 2298-2310.
-
Pateyjohns IR, Brinkworth GD, Buckley JD, Noakes M, Clifton PM (2006) Comparison of three bioelectrical impedance methods with DXA in overweight and obese men. Obesity (Silver Spring) 14: 2064-2070.
-
Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM (2007) Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr 26: 771-777.
-
Shafer KJ, Siders WA, Johnson LK, Lukaski HC (2009) Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass indexes. Nutrition 25: 25-32.
-
Jager-Wittenaar H, Dijkstra PU, Earthman CP, Krijnen WP, Langendijk JA, et al. (2014) Validity of bioelectrical impedance analysis to assess fat-free mass in patients with head and neck cancer: an exploratory study. Head Neck 36: 585-591.
-
Varady KA, Santosa S, Jones PJ (2007) Validation of hand-held bioelectrical impedance analysis with magnetic resonance imaging for the assessment of body composition in overweight women. Am J Hum Biol 19: 429-433.
-
Browning LM, Mugridge O, Chatfield MD, Dixon AK, Aitken SW, et al. (2010) Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity (Silver Spring) 18: 2385-2391.
-
Otto M, Farber J, Haneder S, Michaely H, Kienle P, et al. (2015) Postoperative Changes in Body Composition-Comparison of Bioelectrical Impedance Analysis and Magnetic Resonance Imaging in Bariatric Patients. Obes Surg 25: 302-309.
-
Thomas EL, Collins AL, McCarthy J, Fitzpatrick J, Durighel G, et al. (2010) Estimation of abdominal fat compartments by bioelectrical impedance: the validity of the ViScan measurement system in comparison with MRI. Eur J Clin Nutr 64: 525-533.
-
Bracco D, Thiebaud D, Chiolero RL, Landry M, Burckhardt P, et al. (1996) Segmental body composition assessed by bioelectrical impedance analysis and DEXA in humans. J Appl Physiol 81: 2580-2587.
-
Ludbrook J (2002) Statistical techniques for comparing measurers and methods of measurement: a critical review. Clin Exp Pharmacol Physiol 29: 527-536.
-
Sun G, French CR, Martin GR, Younghusband B, Green RC, et al. (2005) Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr 81: 74-78.
-
Deurenberg P, Andreoli A, Borg P, Kukkonen-Harjula K, de Lorenzo A, et al. (2001) The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr 55: 973-979.
-
Yamakage H, Ito R, Tochiya M, Muranaka K, Tanaka M, et al. (2014) The utility of dual bioelectrical impedance analysis in detecting intra-abdominal fat area in obese patients during weight reduction therapy in comparison with waist circumference and abdominal CT. Endocr J 61: 807-819.





