Clinical Medical
Reviews and Case Reports
Interferon and Ribavirin-Induced Oral Hyperpigmentation in Two Taiwanese Patients: Case Report and Literature Review
Yu-Jong Weng1,2* and Min-Fu Tsan3
1Department of Medicine, Taichung Veterans General Hospital, Republic of China
2Department of Applied Chemistry, National Chia-Yi University, Republic of China
3McGuire Research Institute and Veterans Affairs Medical Center, USA
*Corresponding author: Yu-Jong Weng, Division of Gastroenterology, Department of Medicine, Taichung Veterans General Hospital, 600, Setion 2, Shixian Road, West District, Chia-Yi Branch, Chia-Yi, Taiwan 60090, Republic of China, Tel: +886-(0)5-2359630 x 1002, Fax: +886-(0)5-2369676, E-mail: yujong3495@gmail.com
Clin Med Rev Case Rep, CMRCR-2-067, (Volume 2, Issue 11), Case Report; ISSN: 2378-3656
Received: August 03, 2015 | Accepted: October 28, 2015 | Published: November 01, 2015
Citation: Yu-Jong W, Min-Fu T (2015) Interferon and Ribavirin-Induced Oral Hyperpigmentation in Two Taiwanese Patients: Case Report and Literature Review. Clin Med Rev Case Rep 2:067. 10.23937/2378-3656/1410067
Copyright: © 2015 Yu-Jong W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Hyperpigmentation of the tongue and oral mucosa is a rare adverse event of the combination therapy with either interferon α (IFN) or polyethylene glycol-conjugated IFN (PEG-IFN) and ribavirin (RBV) in patients with hepatitis C virus (HCV) infection. The majority of these lesions either improves or resolves completely after completion of the therapy. It occurs more frequently in patients with dark skins and in female patients. While most patients have tongue hyperpigmentation alone, others also have hyperpigmentation involving gum, hard palate and/or buccal mucosa. It is not clear whether the hyperpigmentation of tongue and/or oral mucosa is associated with similar lesions in the gastrointestinal mucosa. We reported the first two cases of PEG-IFN and RBV combination therapy-induced oral hyperpigmentation in Taiwanese patients with hepatitis C, one in the tongue and the other in the buccal mucosa. Upper gastrointestinal (UGI) endoscopic exams revealed that none of these two patients had similar hyper-pigmented lesions in the esophageal, gastric, and duodenal mucosa. Since none of the previous reports mentioned any UGI endoscopic findings, further studies are necessary to determine whether the IFN or PEG-IFN and RBV-induced hyperpigmentation may also involve UGI mucosa. The mechanism of IFN and RBV-induced oral hyperpigmentation remains unclear. While most authors attributed oral hyperpigmentation to IFN or PEG-IFN, there has been no reported case of oral hyperpigmentation in patients treated with IFN, PEG-IFN or RBV alone. Therefore, it is more appropriate to call it IFN or PEG-IFN and RBV-induced oral hyperpigmentation.
Keywords
Oral hyperpigmentation, Interferon, Polyethylene glycol-conjugated interferon, Ribavirin, and Hepatitis C
Introduction
A variety of medications such as non-steroidal anti-inflammatory drugs, anti-malarials, amiodarone, cytotoxic drugs, tetracyclines, heavy metals, and psychotropic drugs, may cause cutaneous hyperpigmentation [1]. Recently, hyperpigmentation of the tongue and oral mucosa have also been reported in patients with hepatitis C virus (HCV) infection who were being treated with either short acting IFN or long acting polyethylene glycol-conjugated IFN (PEG-IFN) combined with ribavirin (RBV) [1,2]. The IFN or PEG-IFN and RBV-induced oral hyperpigmentation appears to be more common in patients with dark skin, even though it has been reported in both Caucasian as well as non-Caucasian patients [3-5]. It is not clear whether IFN and ribavirin-induced oral hyperpigmentation is associated with similar lesions in the gastrointestinal mucosa in the affected patients. Here we report oral hyperpigmentation developed during the PEG-IFN and RBV combination therapy in two Taiwanese patients with hepatitis C. Upper gastrointestinal (UGI) endoscopic exams revealed that no similar hyper-pigmented lesions were noted in the esophageal, gastric, and duodenal mucosa.
Case report
Case 1
A 33-year-old Taiwanese male patient with HCV infection developed hyperpigmentation of his tongue 18 weeks after the initiation of PEG-IFN α-2b (180 μg weekly) plus RBV (1,200 mg daily) therapy. On physical examination, irregular brown, macular, pigmented patches were noted on ventral and dorsolateral aspects of his tongue (Figure 1A). No hyper-pigmented lesions were noted in his gum, hard palate, inner side of cheeks, nails or other cutaneous sites. There was no discomfort or altered taste associated with his tongue hyperpigmentation. His HCV genotype was Ia and his HCV ribonucleic acid (RNA) level before therapy was 3,357,596 IU/ml. He was not taking any medications or having any medical conditions that might cause hyperpigmentation [1,6,7]. He complained of acid regurgitation and substernal chest pain. An UGI endoscopic exam revealed esophageal mucosal break without evidence of mucosal hyperpigmentation in esophagus, stomach, and up to the 2nd portion of the duodenum. He was treated with proton pump inhibitor with prompt resolution of the chest pain. Six months after the completion of a 24-week PEG-IFN and RBV therapy, there was no improvement in his pigmented tongue lesions.
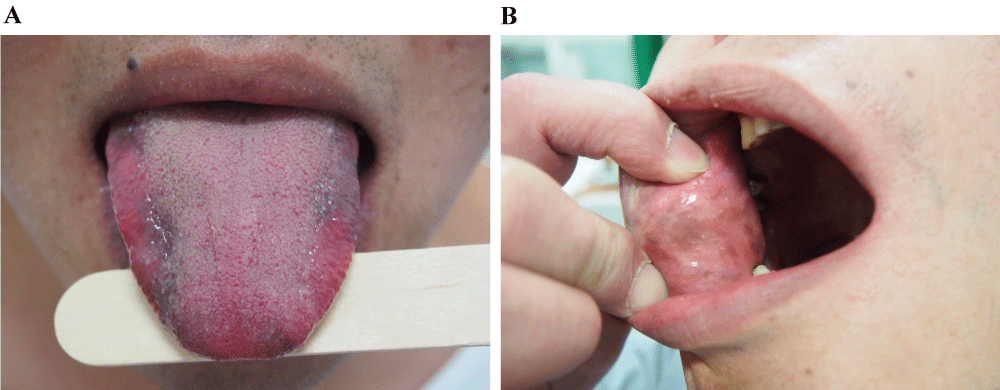
.
Figure 1A: Case 1: Hyper-pigmented patches on the dorsolateral aspects of the tongue
Figure 1B: Case 2: Hyper-pigmented patches on inner side of bilateral cheeks
View Figure 1
Case 2
A 31-year-old Taiwanese male patient with HCV and human immunodeficiency virus (HIV) co-infection was treated with a combination of PEG-IFN α-2b (180 μg weekly) plus RBV (1,200 mg daily) for his hepatitis C. Twenty nine weeks after initiation of the therapy; he noted sensitive hyper-pigmented patches on his bilateral buccal mucosa (Figure1B). On physical exam, no similar lesion was found in his tongue, gum, hard palate, nail or other cutaneous sites. His HCV genotype was Ia and his HCV RNA level was 3,679,809 IU/ml before therapy. He was not taking any medications or having any medical conditions that might cause hyperpigmentation [1,6,7]. An UGI endoscopic exam was performed due to complaint of acid regurgitation and heartburn. Antral gastritis was found, however, there was no similar mucosal hyperpigmentation in esophagus, stomach, and proximal duodenum. Two months after the completion of a 48-week PEG-IFN and RBV therapy, there was no improvement in his pigmented tongue lesions.
Discussion
A total of 30 cases of IFN or PEG-IFN and RBV- induced oral hyperpigmentation have been reported in the literature since 2003 (summarized in table 1) [2-5,8-21]. The incidence of this side effect is not clear. Three retrospective studies revealed an incidence of 2.9%, i.e., 5 out of 171 patients [3], 0.3%, i.e., 1 out of 286 patients [19], and 0.7%, i.e., 1 out of 152 patients [21]. However, a prospective study, which only included patients who had received the PEG-IFN/RBV therapy for more than 3 months, showed a much higher incidence of 9.9%, i.e., 7 out of 77 patients [4]. The incidence would be even higher, i.e., 21% or 16 out of 77 patients, if one included all patients with PEG-IFN/RBV-induced hyperpigmentation, i.e., tongue, oral mucosa, nail and skin [4]. The reason for the discrepancy in these studies is not clear.
![]()
Table 1: Characteristics of IFN or PEG-IFN and RBV-induced tongue and oral mucosa hyperpigmentation
View Table 1
Analysis of these 30 reported cases revealed that IFN or PEG-IFN and RBV-associated oral hyperpigmentation affected predominantly dark skinned patients (21 of 30 patients). Of the 23 patients whose sex was available in the reports, 15 patients were female with a female/male ratio of 1.9. Oral hyperpigmentation might occur as quickly as 1 month or as late as 12 months after the initiation of IFN or PEG-IFN and RBV therapy. The most frequent site of oral hyperpigmentation was the tongue (100%). While most patients had tongue hyperpigmentation alone (16 patients), others also had hyperpigmentation involving gum, hard palate and/or buccal mucosa. Eight patients also had lesions involving other parts of the body skin. Interestingly, in 5 of the 30 cases nails (melanonychia) were also involved [4], and 1 female patient also had vulvar mucosa hyperpigmentation [20]. The majority of these patients did not have any symptoms associated with this IFN- or PEG-IFN and RBV-induced oral pigmentation. Only 7 patients reported tongue symptoms, such as pain, discomfort, burning sensation, or sensitivity to hot and spicy food. None of these patients required reduction in IFN dosages or discontinuation of the IFN therapy. The lesions disappeared in 3 patients, improved in 9 patients, and unchanged in 7 patients at various intervals, i.e., 2-24 months, after completion of the IFN or PEG-IFN and RBV therapy. In one patient the tongue lesion disappeared during the therapy. Whether these differences in outcomes were due to the different length of patient follow-up is not clear.
The mechanism for the IFN or PEG-IFN and RBV therapy-induced oral hyperpigmentation is not clear. Most authors attribute it to IFN or PEG-IFN, because it has been shown that IFN up-regulates the expression of α-melanocyte stimulating hormone receptors in murine melanocytes, which may lead to an increased melanin production [22]. However, there has been no report of oral hyperpigmentation in patients who were treated with either IFN or RBV alone. Thus, until there is convincing evidence to demonstrate that IFN or PEG-IFN alone can induce oral hyperpigmentation, it is prudent to say that it is the combination of IFN or PEG-IFN and RBV that induced the oral hyperpigmentation.
Clinical diagnosis of IFN or PEG-IFN and RBV-induced oral hyperpigmentation appears straightforward, when hyperpigmentation of tongue and/or oral mucosa develops in a patient during the course of IFN or PEG-IFN and RBV combination therapy. However, it is necessary to rule out other concomitant medications or other medical conditions, such as melanoma, acanthosis nigricans, hemochromatosis and Addison's disease, that may cause systemic hyperpigmentation [1,6,7]. Immunohistochemical examination of these hyperpigmented lesions reveals an increased deposit of melanin, confirming the presence of hyperpigmentation [2]. However, biopsy of the lesion is usually not required for the clinical diagnosis of IFN or PEG-IFN and RBV-induced oral hyperpigmentation.
In the current report, we presented 2 additional cases of PEG-IFN and RBV-induced oral hyperpigmentation, one involving the tongue, while the other involving the buccal mucosa alone. Hyperpigmentation involving buccal mucosa without tongue involvement has not been described previously. In addition, we noted that no similar hyper-pigmented lesions were noted in the esophageal, gastric, or duodenal mucosa upon UGI endoscopic exams in these 2 patients. Since none of the previously reported cases had mentioned any UGI endoscopic findings, it is not clear whether the IFN or PEG-IFN and RBV-induced hyperpigmentation may also involve UGI mucosa.
In summary, IFN or PEG-IFN and RBV-induced oral hyperpigmentation is a benign side effect requiring no need to reduce the dosage or to discontinue the therapy. The majority of these lesions will either improve or disappear after completion of the therapy. It occurs probably more common in patients with dark skins and female patients. HCV infection is a global health problem affecting 130 to 170 million individuals worldwide. The recent development of direct-acting antiviral agents may revolutionize the therapy of HCV infection [23]. However, due to the high cost of these newer agents, PEG-IFN and RBV will continue to be the main stay for the treatment of hepatitis C, particularly in resource-limited countries. Clinicians should be familiar with the possibility of PEG-IFN and RBV-induced oral hyperpigmentation.
Acknowledgement
None
Ethical Statement
This case report was not a research involving human subjects and no identifiable individual information was collected or reported, therefore no Institutional Review Board approval is required.
Conflict of Interest
The authors declare no real or perceived conflict of interest related to the content of this manuscript.
References
-
Dereure O (2001) Drug-induced skin pigmentation. Epidemiology, diagnosis and treatment. Am J Clin Dermatol 2: 253-262.
-
Willems M, Munte K, Vrolijk JM, Den Hollander JC, Bohm M, et al. (2003) Hyperpigmentation during interferon-alpha therapy for chronic hepatitis C virus infection. Br J Dermatol 149: 390-394.
-
Gurguta C, Kauer C, Bergholz U, Formann E, Steindl-Munda P, et al. (2006) Tongue and skin hyperpigmentation during PEG-interferon-alpha/ribavirin therapy in dark-skinned non-Caucasian patients with chronic hepatitis C. Am J Gastroenterol 101: 197-198.
-
Tsilika K, Tran A, Trucchi R, Pop S, Anty R, et al. (2013) Secondary hyperpigmentation during interferon alfa treatment for chronic hepatitis C virus infection. JAMA Dermatol 149: 675-677.
-
Torres HA, Bull L, Arduino RC, Barnett BJ (2007) Tongue hyperpigmentation in a caucasian patient coinfected with HIV and hepatitis C during peginterferon alfa-2b and ribavirin therapy. Am J Gastroenterol 102: 1334-1335.
-
Stulberg DL, Clark N, Tovey D (2003) Common hyperpigmentation disorders in adults: Part I. Diagnostic approach, cafe au lait macules, diffuse hyperpigmentation, sun exposure and phototoxic reactions. Am Fam Physician 68: 1955-1960.
-
Stulberg DL, Clark N, Tovey D (2003) Common hyperpigmentation disorders in adults: Part II. Melanoma, seborrheic keratosis, acanthosis nigricans, melisma, diabetic dermopathy, tinea vesicolor, and postinflammatory hyperpigmentation. Am Fam Physician 68: 1963-1968.
-
Sood A, Midha V, Bansal M, Goyal A, Sharma N (2006) Lingual hyperpigmentation with pegylated interferon and ribavirin therapy in patients with chronic hepatitis C. Indian J Gastroenterol 25: 324.
-
Radha Krishna Y, Itha S (2010) What caused this lingual hyperpigmentation in a patient with chronic hepatitis C? Liver Int 30: 416.
-
Dell'Isola S, Ialungo AM, Starnini G, Roselli P, Ghittoni G, et al. (2008) A surprising hyperpigmentation of the gums and tongue. Gut 57: 1697-1727.
-
Fernandez A, Vazquez S, Rodriguez-Gonzalez L (2008) Tongue hyperpigmentation resulting from peginterferon alfa-2a and ribavirin treatment in a Caucasian patients with chronic hepatitis C. J Eur Acad Dermatol Venereol 22: 1389-1390.
-
de Moraes PC, Noce CW, Thomaz LA, Mautoni MC, Correa ME (2009) Tongue hyperpigmentation resulting from peginterferon alfa and ribavirin combination therapy: a case report. J Am Dent Assoc 140: 1377-1379.
-
Aguayo-Leiva I, Perez B, Salguero I, Jaen P (2009) Tongue hyperpigmentation during interferon-alpha and ribavirin therapy. Eur J Dermatol 19: 291-292.
-
Farshidi D, Chiu MW (2010) Lingual hyperpigmentation from pegylated interferon and ribavirin treatment of hepatitis C. J Am Acad Dermatol 62: 164-165.
-
Karabay O, Goksugur N, Ogutlu A (2011) Tongue hyperpigmentation during interferon therapy. J Dermatol 38: 290-291.
-
Ghosh S, Duseja A, Dhiman RK, Chawla YK (2012) Tongue hyperpigmentation resulting from peginterferon alfa-2b and ribavirin treatment in a patient with chronic hepatitis C. Dig Dis Sci 57: 820-821.
-
Bachmeyer C, Pellen JC (2012) Tongue hyperpigmentation during hepatitis C treatment. CMAJ 184: 1498.
-
Mlika RB, Kerkeni N, Marrak H, Fenniche S, Mokhtar I, et al. (2013) Tongue hyperpigmentation during PEG-interferon-alfa/ribavirin therapy in a non-Caucasian patient with chronic hepatitis C: a case report and review of literature. Int J Dermatol 52: 643-644.
-
Tavakoli-Tabasi S, Bagree A (2012) A longitudinal cohort study of mucocutaneous drug eruptions during interferon and ribavirin treatment of hepatitis C. J Clin Gastroenterol 46: 162-167.
-
Marcoval J, Notario J, Martin C, Gomez S (2014) Oral hyperpigmentation associated with interferon-alpha and ribavirin therapy for hepatitis C virus infection. Actas Dermosifiliogr 105: 211-212.
-
Li Z, Zhang Y, An J, Feng Y, Deng H, et al. (2014) Predictive factors for adverse dermatological events during pegylated/interferon alpha and ribavirin treatment for hepatitis C. J Clin Virol 60: 190-195.
-
Kameyama K, Tanaka S, Ishida Y, Hearing VJ (1989) Interferons modulate the expression of hormone receptors on the surface of murine melanoma cells. J Clin Invest 83: 213-221.
-
Liang TJ, Ghany MG (2014) Therapy of hepatitis C--back to the future. N Engl J Med 370: 2043-2047.





