International Journal of Neurology and Neurotherapy
Depression in Parkinson's Disease is Associated with a Serotoninergic System Change Secondary to Neuroinflammation
Ronise M Santiago1*, Maria ABF Vital1, Marcelo D O Sato2 and Gustavo P Adam3
1Department of Pharmacology, Federal University of Parana, Brazil
2Pharmacology and Physiology, Faculdade Evangelica do Parana, Brazil
3Psychiatrist of City hall of Curitiba, Brazil
*Corresponding author:
Ronise Martins Santiago, Departamento de Farmacologia, Universidade Federal do Parana, Brazil, Tel: +55-41-3361-1717, Fax: +55-41-3266-2042, E-mail: ronise.santiago@gmail.com
Int J Neurol Neurother, IJNN-3-061, (Volume 3, Issue 6), Research Report; ISSN: 2378-3001
Received: July 20, 2016 | Accepted: November 28, 2016 | Published: December 03, 2016
Citation: Santiago RM, ABF Vital M, Sato MDO Adam GP (2016) Depression in Parkinson's Disease is Associated with a Serotoninergic System Change Secondary to Neuroinflammation. Int J Neurol Neurother 3:061. 10.23937/2378-3001/3/6/1061
Copyright: © 2016 Santiago RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Depression is a common psychiatric disorder in patients with Parkinson's disease (PD), being more prevalent in PD than in any other chronic disabling disease. Its cause, nevertheless, has not yet been elucidated. According to some authors, there is a decrease in serotonin (5-HT) levels compensatory to dopaminergic release impairment, but new evidence suggests that chronic inflammation may be a more likely etiogenic factor to depression. The inflammatory hypothesis states that depression is caused by an activation of the inflammatory system, incrementing production of proinflammatory cytokines such as prostaglandin (PG) E2, and these cytokines promote changes in the metabolic pathway of tryptophan, reducing its levels and, hence, the availability of 5-HT. This establishes an important link between the levels of inflammatory cytokines and monoamines. We consider the hypothesis that depression in PD is associated with reduced serotonin levels by neuroinflammation. In conclusion, we suggest that depression associated with PD is related to a change in the serotonergic system caused by neuroinflammation.
Keywords
Depression, Parkinson's disease, Neuroinflammation
Introduction
Depression (depressive disorder) is a common psychiatric disorder in patients with Parkinson's disease (PD), being more prevalent in PD than in any other chronic disabling disease. [1]. The prevalence of depression in PD patients is higher than 40% [2], and its incidence reaches 1.86% per year [3,4]. Despite they being important comorbidities, the cause of depression in PD remains unknown. Our hypothesis is that depression in PD is caused by a decrease in central serotonin (5-hydroxytryptamine, 5-HT) levels, secondary to neuroinflammation.
Depression in Parkinson's Disease
The dopaminergic and serotonergic neurotransmitter systems are involved in regulating mood, and changes in these systems are associated with depression in the general population and in patients with neurodegenerative diseases like PD [5]. According to some authors, the 5-HT decrease is a compensatory mechanism associated with reduction in dopaminergic neurotransmission. Serotonin has an inhibitory function in DA release in the striatum [1,6]. Politis, et al. [7] reported that the occurrence of non-motor symptoms in PD patients may be associated with loss of serotonergic neurons.
The levels of 5-HT and its metabolite 5-HIAA in cerebrospinal fluid (CSF) are also reduced in depressed patients [8]. Other studies demonstrated that CSF levels of 5-HIAA in PD patients are lower than in healthy individuals [9-11] and this reduction is even larger when comparing PD plus depression to PD without depression [12]. In a previous study, our group showed that the depressive-like behaviors observed in Parkinsonian rats produced through the intranigral infusion of 6-hydroxydopamine (6-OHDA) was correlated with a reduction in the levels of hippocampal 5-HT and its metabolite [13]. We demonstrate, also, that the depressive-like behaviors are observed from day 7 until day 21 after infusion of 6-OHDA, and that the reduction of hippocampal 5-HT occurred on the 1st day after the lesion with 6-OHDA in the SN and persisted until 21 days after infusion [14].
Attempting to explain the cause of depression in PD patients, new approaches were proposed, as the inflammatory hypothesis of depression [12]. Evidence suggests that continuous activation of the immune system and/or chronic inflammation system can be one of the pathological processes associated with PD depression [15]. It is known that the formation of Lewy corpuscle, pathological feature of PD, and the death of dopaminergic neurons, promotes activation of microglia, which produces neurotoxic factors, such as cytokines [16], and increased expression of cyclooxygenase (COX-2) thus increasing the synthesis of prostaglandin (PG) [17]. In PD patients, the levels of inflammatory cytokines are increased in the substantia nigra pars compacta (SNpc), striatum and CSF [18,19]. According to Maes, et al. [20], dysfunction in serotonergic system in depression is the result of cell-mediated immune activation.
Neuroinflammation, depression and parkinson's disease
Inflammation is the first line of defense against tissue injury or infection, but an excessive inflammatory response can cause tissue damage. Neurons, as a result of its large cell differentiation, have little or no ability to divide and a reduced ability to recovery from injury, becoming, therefore, extremely vulnerable to autoimmune and inflammatory processes [21]. According to Collins, et al. [22], in PD, when the glial cells are activated, they secrete high levels of proinflammatory mediators that may induce dopaminergic neurons death, that further increase the activation of glial cells, resulting in a vicious cycle of neurodegeneration and inflammation. Besides that, the neuronal COX-2 expression is related to apoptosis and involved in the response to stress [23].
Initial inflammatory response in innate immunity sparks a cascade that results in the activation and recruitment of adaptive immunity [16]. Therefore, proinflammatory cytokines released during the innate and adaptive immune responses can lead to the development of central nervous system disorders [24]. Stress conditions are associated with an increase in hypothalamic-pituitary-adrenal (HPA) axis activity and elevated serum corticosterone [25,26]. Studies support, also, the idea that activation of the HPA axis in response to chronic stress induces changes in the hippocampal serotonergic system, predisposing the individual to the development of depression [27,28].
Patients with depression and PD have an increase in the inflammatory processes, with increased levels of interleukin 1β, interleukin 6, tumor necrosis factor (TNF)-α and cortisol [5]. Depression patientes also have increased concentration of proinflammatory cytokines and PGE2 in blood and CSF [29-32]. Evidence suggests that depression increases neuroinflammation and that the antidepressants exert anti-inflammatory effects [33,34]. Studies in animals and humans indicate that cytokines interact with many pathophysiological grounds that characterize depression as the metabolism of neurotransmitters, synaptic plasticity and neuroendocrine function [35-37].This hypothesis is corroborated, yet, by the presence of activated microglia, increased proinflammatory cytokines and COX-2 in neuronal and glial post-mortem PD patients' brains [18,38]. The expression of COX-2 has been associated with the degeneration of dopaminergic neurons of the SNpc in both humans and in animal PD models [39,40]. Patients suffering from chronic inflammatory processes are at increased risk to develop depression, as well as patients treated with proinflammatory cytokines such as interleukins, interferons (IFN)-γ or TNF-α [41,42].
On the other hand, these cytokines promote changes in the metabolic pathway of tryptophan reducing levels and hence the availability of 5-HT. Proinflammatory cytokines are able to reduce the level of 5-HT through the activation of indoleamine 2, 3-dioxygenase (IDO) [43]. IDO is an enzyme that degrades tryptophan to a catabolite of tryptophan (TRYCATs) and nicotinamide, reducing the bioavailability of tryptophan for the synthesis of 5-HT [44]. The neurotransmitter 5-HT is derived from an essential amino acid, tryptophan, and its synthesis in the brain is highly dependent on the bioavailability of plasma tryptophan [45], so that the activation of IDO by proinflammatory cytokines, induces the tryptophan metabolism reducing its bioavailability and, consequently, the level of 5-HT in neurons [46]. Not only cytokines, but PGE2 is also able to reduce the level of 5-HT through the activation of IDO enzyme [41,47-49].
Anti-inflammatories as antidepressants in parkinson's disease
The pharmacological mechanism of action of nonsteroidal anti-inflammatory drugs (NSAIDs) arises from inhibition of COX, the limiting step enzyme for the synthesis of PGs from arachidonic acid [50]. COX is presented in two isoforms - a constitutive isoform (COX-1) and an inducible isoform (COX-2), both sharing a high degree of structural homology. However, COX isoforms are so pharmacologically distinct, that they are differentially inhibited by NSAIDs [51]. Traditional NSAIDs and aspirin (ASA) inhibit both isoforms and selective NSAIDs (coxibs) preferentially inhibit COX-2 [50]. Studies suggest that both isoforms are unevenly distributed among cells of the central nervous tissue, COX-1 being detected in microglial cells and COX-2 found primarily in glial cells, and at lower levels (above subject to regulation in patients PD and induction models of parkinsonism), in the dopaminergic neurons of the SNpc [52].
The investigation of potentially neuroprotective effect of NSAIDs in PD experimental models was instigated by in vitro studies developed by Grilli, et al. [53], who demonstrated that aspirin and sodium salicylate were able to prevent glutamate-induced neurotoxicity, suggesting a potential neuroprotective effect of those drugs. In a parecoxib study, it was demonstrated that this drug has been effective in stopping PD motor and cognitive deficit and changes in the expression of tyrosine hydroxylase (TH) caused by intranigral infusion of 1,2,3,6-tetrahydropyridine (MPTP) in rats [54]. In the same line of work, Soliman, et al. [55] demonstrated that administration of piroxicam 7 days before and after the administration of MPTP (40 mg/kg) in mice was able to reduce neurodegeneration in SNpc and consequent motor disabilities. Using unilateral infusion of 6-OHDA in rats, Pernaute-Sanchez, et al. [56] evaluated the neuroprotective effect of chronic treatment with celecoxib, a selective inhibitor of COX-2, and the results demonstrated neuroprotection, reduced microglial activation and increased immunoreactivity for TH. In addition, the chronic treatment with celecoxib was also able to reverse the depressive-like behavior induced by moderate chronic stress protocol in rats, reducing the expression of COX-2 in the brain and, subsequently, the concentration of PGE2 [57].
Hunter, et al. [58] observed that celecoxib limited the inflammatory response induced by LPS by reducing the release and overproduction of cytotoxic molecules and partially restoring mitochondrial function, thus increasing dopaminergic neuronal survival. According to Bartels and Leenders [59], the observed benefit of celecoxib may also stem directly from COX-2 neuronal inhibition, which is apparently able, directly and through the inhibition of microglial activation, to reduce dopaminergic neurodegeneration.
Kohler, et al. [60] described in their recent meta-analysis that treatment with non-steroidal anti-inflammatory drugs improved depressive symptoms in PD in 9 out of the 10 studies evaluated without increasing the risk of adverse effects. Mendlewicz, et al. [61] demonstrated that PD patients treated with ASA, in addition to their antidepressant therapy, showed improvement in the first week of treatment. In addiction, patients with PD and depression treated with celecoxib adjunctive to the antidepressant drug demonstrated improvement in the Hamilton scale for Depression scores [62-65]. In conclusion, adjuvant treatment with NSAIDs may be a promising strategy for patients with depressive disorder and PD [65,66].
Finally, drugs that inhibit proinflammatory cytokine signaling such as NSAIDs represent a viable strategy for the treatment of depression, especially in patients with evidence of increased inflammatory activity as in PD [67]. According to Menza, et al. [68] inflammatory cytokines may be involved in the neurobiology of initiation and/or maintenance of depression in PD, supporting the hypothesis that neuroinflammation is involved in the depression secondary to PD.
Conclusion
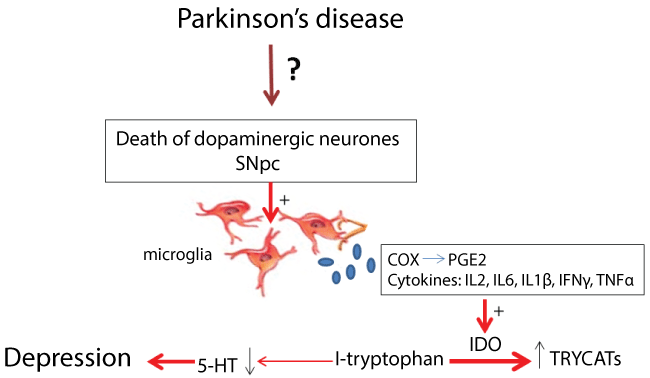
In conclusion, we believe that depression associated with PD is related to alteration in the serotonergic system caused, in part, by a neuroinflammatory process. The inflammatory process leads to release of cytokines and PG that are able to activate IDO and thus reduce the availability of central 5-HT, leading, therefore to the onset of depression, as shown in (Figure 1).
.
Figure 1: Scheme proposed for the cause of depression in Parkinson's disease. SNpc: substantia nigra compact part COX: cyclooxygenase; PGE2: prostaglandin E2; IL: interleukin; IFNγ: interferon gamma; TNFα: tumor necrosis factor alpha; IDO: indoleamine 2 3-dioxygenase; TRYCATs: catabolites of tryptophan; 5-HT: serotonin.
View Figure 1
Acknowledgements
This work was supported by grants from CNPq, CAPES and the Araucária Foundation, which had no further role in the study design, collection, analysis, and writing the report, or decision to submit the manuscript for publication. MABFV is recipients of CNPq fellowships.
References
-
Kanda F, Oishi K, Kuga A, Kobessho H, Shirafuji T, et al. (2008) Characteristics of depression in Parkinson's disease: Evaluating with Zung's Self-Rating Depression Scale. Parkinsonism and Related Disorders 14: 19-23.
-
Cummings JL (1992) Depression and Parkinson's disease: a review. Am J Psychiatry 149: 443-454.
-
Althaus A, Becker OA, Spottke A, Dengler R, Schneider F, et al. (2008) Frequency and treatment of depressive symptoms in a Parkinson's disease registry. Parkinsonism Relat Disord 14: 626-632.
-
Frisina PG, Haroutunian V, Libow LS ( 2009) The neuropathological basis for depression in Parkinson's disease. Parkinsonism Relat Disord 15: 144-148.
-
Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P (2011) Depression in Parkinson disease-epidemiology mechanisms and management. Nat Rev Neurol 8: 35-47.
-
Suzuki K, Miyamoto M, Miyamoto T, Okuma Y, Hattori N, et al. (2009) Correlation between depressive symptoms and nocturnal disturbances in Japanese patients with Parkinson's disease. Parkinsonism Related Disord 15: 15-19.
-
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, et al. (2012) Serotonin neuron loss and nonmotor symptoms continue in Parkinson's patients treated with dopamine grafts. Sci Transl Med 4: 128-41.
-
Tan SKH, Hartung H, Sharp T, Temel Y (2011) Serotonin-dependent depression in Parkinson's disease: A role for the subthalamic nucleus?. Neuropharmacology 61: 387-399.
-
Johansson B, Roos BE (1967) 5-Hydroxyindoleacetic and homovanillic acid levels in the cerebrospinal fluid of healthy volunteers and patients with Parkinson's syndrome. Life Sci 6: 1449-1454.
-
Gottfries CG, Gottfries I, Roos BE (1969) Homovanillic acid and 5-hydroxyindoleacetic acid in the cerebrospinal fluid of patients with senile dementia presenile dementia and parkinsonism. J Neurochem 16: 1341-1345.
-
Chase TN, Ng LK, Watanabe AM (1972) Parkinson's disease. Modification by 5-hydroxytryptophan. Neurology 22: 479-484.
-
Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, et al. (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis 24: 27-53.
-
Santiago RM, Barbieiro J, Lima MMS, Dombrowski PA, Andreatini R, et al. (2010) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson's disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry 34: 1104-1114.
-
Santiago RM, Barbiero J, Gradowski RW, Boschen S, Lima MM, et al. (2014) Induction of depressive-like behavior by intranigral 6-OHDA is directly correlated with deficits in striatal dopamine and hippocampal serotonin. Behav Brain Res 259: 70-77.
-
Currier MB, Nemeroff CB (2010) Inflammation and mood disorders: proinflammatory cytokines and the pathogenesis of depression. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry 9: 212-220.
-
Mosley RL, Benner EJ, Kadiu I, Thomas M, Bosa MD, et al. (2006) Neuroinflammation oxidative stress and the pathogenesis of Parkinson's disease. Clinical Neuroscience Research 6: 261-281.
-
Hald A, Lotharius J (2005) Oxidative stress and inflammation in Parkinson's disease: Is there a causal link?. Exp Neurol 193: 279-290.
-
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Trans 60: 277-290.
-
Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson's Disease. Movement Disorders 28: 24-30.
-
Maes M, Meltzer HY, Scharpe S, Bosmans E, Suy E, et al. (1993) Relationships between lower plasma L-tryptophan levels and immune inflammatory variables in depression. Psychiatry Res 49: 151-165.
-
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson's disease. Trends Pharmacol Sci 24: 395-401.
-
Collins LM, Toulouse A, Connor TJ, Nolan YM (2012) Contributions of central and systemic inflammation to the pathophysiology of Parkinson's disease. Neuropharmacology 62: 2154-2168.
-
McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, et al. (2006) Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci 37: 9365-9375.
-
Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, et al. (2008) Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab 9: 622-7.
-
Palermo-Neto J, de Oliveira Massoco C, Robispierre de Souza W (2003) Effects of physical and psychological stressors on behavior macrophage activity and Ehrlich tumor growth. Brain Behav Immun 17: 43-54.
-
Costa-Pinto FA, Palermo-Neto J (2010) Neuroimune interactions in stress. Neuroimmunomodulation 17: 196-199.
-
Guimaraes FS, Del Bel EA, Padovan CM, Mendonça Netto S, Titze De Almeida R (1993) Hippocampal 5-HT receptors and consolidation of stressful memories. Behav Brain Res 58: 133-139.
-
Joca SRL, Padovan CM, Guimaraes FS (2003) Activation of postsynaptic 5-HT1A receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res 978: 177-184.
-
Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, et al. (1986) Depression immunocompetence and prostaglandins of the E series. Psychiatry Res 17: 41-47.
-
Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, et al. (2005) Increase in interleukin-1beta in late-life depression. Am J Psychiatry 162: 175-177.
-
Kahl KG, Bens S, Ziegler K, Rudolf S, Dibbelt L, et al. (2006) Cortisol the cortisol-dehydroepiandrosterone ratio and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry 59: 667-671.
-
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446-457.
-
Abdel-Salam OME, Nofal SM, El-Shenawy SM (2003) Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol Res 48: 157-165.
-
Vismari L, Alves GJ, Muscara MN, Palermo-Neto J (2012) A possible role to nitric oxide in the anti-inflammatory effects of amitriptyline. Immunopharmacol Immunotoxicol 34: 578-585.
-
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732-741.
-
Loftis JM, Huckans M, Morasco BJ (2010) Neuroimmune mechanisms of cytokine-induced depression: Current theories and novel treatment strategies. Neurobiology of Disease 37: 519-533.
-
Kuan-Pin S (2012) Inflammation in psychopathology of depression: Clinical biological and therapeutic implications. Bio Medicine 2: 68-74.
-
Knott C, Stern G, Wilkin GP (2000) Inflammatory regulators in Parkinson's disease: iNOS lipocortin-1 and cyclooxygenases-1 and -2. Mol Cell Neurosci 16: 724-739.
-
Teismann P, Tieuk Choi DK, Wu DC, Naini A, Hunot S, et al. (2003) Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. PNAS 100: 5473-5478.
-
Lima MMS, Reksidler AB, Zanata SM, Machado HB, et al. (2006) Different parkinsonism models produce a time-dependent induction of COX-2 in the substantia nigra of rats. Brain Res 1101: 117-125.
-
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46-56.
-
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24-31.
-
Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R (2011) The new '5-HT' hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs) both of which contribute to the onset of depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry 35: 702-721.
-
Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, et al. (2004) Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry 61: 765-773.
-
Fernstrom JD (1983) Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev 63: 484-546.
-
Leonard B, Maes M (2012) Mechanistic explanations how cell-mediated immune activation inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36: 764-785.
-
Byrne GI, Lehmann LK, Kirschbaum JG, Borden EC, Lee CM, et al. (1986) Induction of tryptophan degradation in vitro and in vivo: a gamma-interferon-stimulated activity. J Interferon Res 6: 389-396.
-
Braun D, Longman RS, Albert ML (2005) A two-step induction of indoleamine 2 3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood 106: 2375-2381.
-
O'Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, et al. (2009) Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2 3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29: 4200-4209.
-
Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010) Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs aspirin and coxibs) on upper gastrointestinal tract. Best Pract Res Clin Gastroenterol 24: 121-132.
-
Smith ML, Hawcroft G, Hull MA (2000) The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer 36: 664-674.
-
Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, et al. (2007) Nonsteroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol 205: 295-312.
-
Grilli M, Pizzi M, Memo M, Spano P (2007) Neuroprotection by aspirin and sodium salicylate through blockade of NF-B activation. Science 274: 1383-1385.
-
Reksidler AB, Lima MMS, Zanata SM, Machado HB, Da Cunha C, et al. (2007) The COX-2 inhibitor parecoxib produces neuroprotective effects in MPTP-lesioned rats. Eur J Pharmacol 560: 163-175.
-
Soliman Y, Jackson T, Mazzio E, Soliman KFA (2009) The effects of piroxicam in the attenuation of MPP+/MPTP toxicity in vitro and in vivo. Neurochem Res 34: 304-310.
-
Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, et al. (2004) Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation 1: 6.
-
Guo JY, Li CY, Ruan YP, Sun M, Qi XL, et al. (2009) Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol 612: 54-60.
-
Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, et al. (2007) Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigroestriatal system. J Neurochem 100: 1375-1386.
-
Bartels AL, Leenders KL (2007) Neuroinflammation in the Pathophysiology of Parkinson's Disease: evidence from animal models to human in vivo studies with [11C]-PK11195 PET. Mov Disord 22: 1852-56.
-
Koohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, et al. (2014) Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71: 1381-1391.
-
Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, et al. (2006) Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol 21: 227-231.
-
Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, et al. (2009) Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety 26: 607-611.
-
Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, et al. (2008) Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol 23: 87-94.
-
Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, et al. (2006) The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 11: 680-684.
-
Na KS, Lee KJ, Lee JS, Cho YS, Jung HY (2014) Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 48: 79-85.
-
Davis A, Gilhooley M, Agius M (2010) Using non-steroidal anti-inflammatory drugs in the treatment of depression. Psychiatr Danub 22: 49-52.
-
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27: 24-31.
-
Menza M, Dobkin RD, Marin H, Mark MH, Gara M, et al. (2010) The Role of Inflammatory Cytokines in Cognition and Other Non-Motor Symptoms of Parkinson's Disease. Psychosomatics 51: 474-479.





