International Journal of Pathology and Clinical Research
Squamous Morules (Microcarcinoids) in Gastroesophageal Polyps; a Mimicker of Invasive Carcinoma
Safia N Salaria1* and Elizabeth Montgomery2
1Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, USA
2Department of Pathology, The Johns Hopkins University School of Medicine, USA
*Corresponding author:
Safia N Salaria, MD, 1161 21st Avenue South, Medical Center North-C2104A, Nashville, TN 37232-2561, USA, Tel: 615-343-1949, Fax 615-936-7040, E-mail: Safia.n.salaria@vanderbilt.edu
Int J Pathol Clin Res, IJPCR-1-015, (Volume 1, Issue 1), Review Article; ISSN: 2469-5807
Received: October 13, 2015 | Accepted: November 27, 2015 | Published: November 30, 2015
Citation: Salaria SN, Montgomery E (2015) Squamous Morules (Microcarcinoids) in Gastroesophageal Polyps; a Mimicker of Invasive Carcinoma. Int J Pathol Clin Res 1:015. 10.23937/2469-5807/1510015
Copyright: © 2015 Salaria SN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Colorectal lesions termed squamous morules or microcarcinoids display predominantly squamous and variable endocrine differentiation and are often found in colorectal adenomas with high grade dysplasia thus mimicking invasion. Herein, we describe histopathologic, immunohistochemical classification and clinical correlation of analogous lesions in the esophagus and stomach. We identified five cases (3 men, 2 women) from November 2004-March 2013 of gastric and gastroesophageal polyps with squamous morules. Four of the patients were white. The median age was 70 years (range 59-85 years). Two patients presented with gastrointestinal bleeding, one was undergoing routine screening, and two were followed up for Barrett esophagus (BE) and a history of gastric polyps respectively. Three lesions were gastric, one gastroesophageal (GE), and one patient had both GE and proximal gastric lesions. Four patients had multiple polyps; one had a 2.5 cm polypoid GE lesion. The median size of the polyps was 1.5 cm. All of the gastric squamous morules arose in hyperplastic polyps (HP) (one patient had separate fundic gland polyps). Two HPs showed high grade dysplasia, 1 low grade dysplasia, and 1 erosions and reactive changes. One of the GE lesions was associated with polypoid papillary high grade dysplasia arising in BE, this patient had follow up biopsy 1 month later showing BE with features indefinite for dysplasia. He died in 2009 (5 years) of unknown cause. The remaining patients are alive. The areas of squamous morule formation ranged in size from 1-3 mm. They were multifocal showing a lobular growth pattern connecting directly to the epithelium. Four of the lesions demonstrated pseudolumina. Immunohistochemical analysis was done on all cases. Sixty percent showed nuclear beta catenin, and all demonstrated a low KI67 index (< 2%). Endocrine markers (synaptophysin, chromogranin) were expressed in all of the lesions, and P63 showed focal labeling in two lesions. In summary, the histopathological features and immunohistochemical labeling of GE squamous morules/microcarcinoids are similar to those previously described in the colorectum. GE lesions can be associated with reactive processes and more frequently in a background of dysplasia. Under-recognition of upper gastrointestinal squamous morules provides potential for over diagnosis of invasive carcinoma. Familiarity with the histopathologic pattern and immunoprofile can aid in avoiding this pitfall.
Keywords
Squamous morules, Microcarcinoids
Introduction
Squamous morules and microcarcinoids (MC) are two among numerous terms used to characterize lesions with varying amounts of squamous and neuroendocrine features. The literature describes numerous associations of these lesions with dysplastic and invasive processes distributed throughout various organs [1].
More recently squamous morules have been described in association with large colorectal polyps [2]. In the colon these lesions have a predilection for high-risk adenomas, villous morphology and those with high grade dysplasia [2]. Often the adenomatous epithelium is connected to or in close association with the lesion, hence the term adenoma-microcarcinoid. The proximity of adenoma-microcarcinoids/squamous morules to dysplastic epithelium can lead to a misdiagnosis of invasive carcinoma [2,3].
Herein, we describe analogous lesions arising in the stomach and esophagus. We performed histopathologic and immunohistochemical analysis along with clinical correlation of patient demographics and follow up.
Materials and Methods
We identified five cases (3 men, 2 women) from November 2004-March 2013 of gastric and gastroesophageal polyps with adenoma-microcarcinoids/squamous morules. The study protocol was approved by The Johns Hopkins Institutional Review Board (02-07-19-05e). Of the five cases, four were received in consultation, and one case was from the material accessioned to Johns Hopkins Hospital. Contributing pathologists, clinicians and chart review were consulted for clinical and follow up history.
Unstained slides of formalin fixed paraffin embedded tissue were obtained and manual immunohistochemical staining for p63 (Abcam CAT# ab124762-1:400), synaptophysin (Abcam CAT# ab32127-1:500) , chromogranin (Abcam CAT # ab15160-1:500), β-catenin (BD Bioscience CAT# 610154-1:1000), and Ki- 67 (Vector Labs CAT# VP-K452 -1:100) were carried on 5 μm thick formalin-fixed, paraffin-embedded sections.
In brief, slides were deparaffinized and hydrated. This was followed by heat-induced antigen retrieval. Incubation with the primary antibody using optimized conditions was followed by secondary antibody incubation, detection and counterstaining per the instruction of the antibody manufacturer. Negative controls included sections of study tissues without primary antibody treatment. Positive cytoplasmic or nuclear labeling was assessed as reactivity greater than that of the negative control.
Results
Clinicopathologic information
Squamous morules/microcarcinoids were identified in five cases from gastric and gastroesophageal polypectomy specimens. These data are summarized in table 1. The median patient age was 70 years (59-85 years). There were three men and two women. Four of the patients were Caucasian; the fifth a Pacific Islander.
![]()
Table 1: Clinical features.
View Table 1
Gastrointestinal bleeding was the clinical indication for endoscopy for three patients, Barrett esophagus follow up and a history of gastric polyps were the clinical indications in the remaining two patients respectively.
A majority of the polyps arose in the stomach (n = 3), one arose in the distal esophagus at 30 cm, and one patient had gastric and gastroesophageal junction lesion. Four patients had multiple polyps; one had a single 2.5 cm polypoid gastroesophageal lesion.
The median size of the polyps was 1.5 cm (range, 1-2.3 cm). All of the gastric squamous adenoma-microcarcinoids/squamous morules arose in association with hyperplastic polyps. One patient had concurrent fundic gland polyps. The clinicopathologic characteristics of the polyps associated with the squamous morules/MCs are highlighted in table 2. High grade dysplasia was found in two hyperplastic polyps, a third showed diffuse low grade dysplasia. The fourth case showed hyperplastic polyps with erosions, acute inflammation and reactive epithelial changes; dysplasia was not identified. Polypoid papillary high grade dysplasia arising in a background of Barrett mucosa was present in the esophageal lesion (Figure 1).
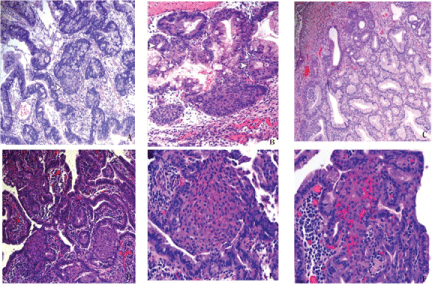
.
Figure 1: Gastroesophageal squamous morules/MCs are often associated with larger dysplastic polyps.
(A) A hyperplastic polyp with areas of high grade dysplasia shows a gastroesophageal microcarinoid connected to the dysplastic epithelium (arrow head). H&E, x10.
(B) The lesions display pseudolumina formation (arrow heads). H&E, x20.
(C) These lesions have also been seen in the setting of reactive changes in hyperplastic polyps. H&E, 10x. (D) An example of gastroesophageal squamous morules/MCs arising in the background of high grade dysplasia in Barrett mucosa, H&E, 10x;
(E) They can show a lobular configuration, H&E, 20x;
(F) A more infiltrative growth pattern, their bland morphology is distinct from the background lesional epithelium, H&E, 20x.
View Figure 1
![]()
Table 2: Clinicopathologic characteristics of polyps.
View Table 2
The adenoma-microcarcinoids/squamous morules were comprised of low cuboidal to squamoid cells with eosinophilic cytoplasm. In addition, some lesions demonstrated intra-cytoplasmic eosinophilic globules. The nuclei were rounded with smooth nuclear borders. The chromatin was fine. Occasional nucleoli were noted. Notably, features of atypia e.g. increased nuclear to cytoplasmic ratio, frequent mitoses, and pleomorphism were absent.
There were several foci of adenoma-microcarcinoids/squamous morules formation in four of the five cases, seen at both the base and surface of the polyps. The median size of the foci of the lesions was 0.2 cm (range, 1-3 cm). The lesions extended over a 1.0 cm area on average. All adenoma-microcarcinoids/squamous morules were connected to the overlying epithelium, in particular two cases showed an epithelial connection at the base of the polyp. Pseudolumina formation was noted in most lesions. All of the foci demonstrated a lobular pattern; in addition three cases showed foci of infiltrative growth. Desmoplastic stromal response was not noted around the lesions nor in the background polyp, there were however, features of mucosal prolapsed (Figure 1). These features are summarized in table 3.
![]()
Table 3: Clinicopathologic characteristics of squamous morules/mcs.
View Table 3
Immunohistochemical analysis revealed that all foci of adenoma-microcarcinoids/squamous morules had a low (less than 2%) Ki-67 index. All of the lesions showed neuroendocrine differentiation by labeling for either synaptophysin (4/5) or chromogranin (1/5). Squamous differentiation by way of p63 nuclear labeling was noted in two cases. A nuclear staining pattern of β-catenin was noted in three cases, cytoplasmic labeling was seen in one case, and the lesional areas were lost on subsequent levels in the one case (Figure 2 and Figure 3). Table 4 highlights the immunohistochemical profiles of the squamous morules/MC.
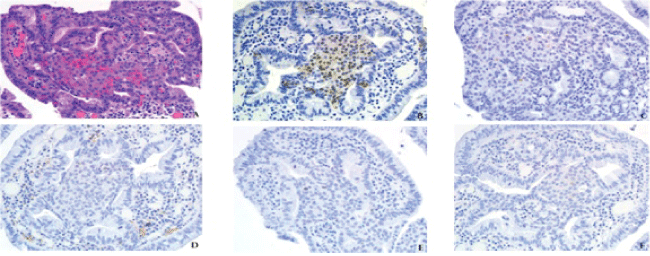
.
Figure 2: (A) Eosinophilic hyaline globules, a feature of gastroesophageal squamous morules/MCs. The growth pattern in this example is infiltrative. H&E, 40x.
(B) β-catenin 40x, (C) Synaptophysin, 40x, (D) Chromogranin, (40x), (E) Ki-67 40x, and (F) p63, 40x.
View Figure 2
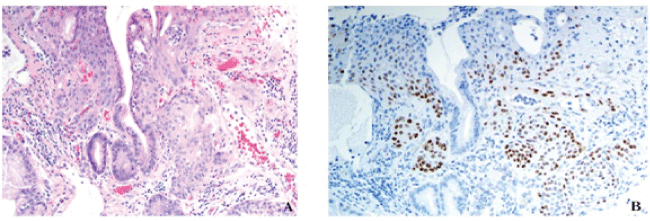
.
Figure 3: Gastroesophageal squamous morules/MC's are also seen in polyps with inflammation and reactive changes. (A) This example demonstrates a gastroesophageal microcarcinoid adjacent to an area of ulceration and acute inflammation. H&E, 20x. (B) p63 20x.
View Figure 3
Follow up data
Pathology follow up data was available on one patient who had initially been diagnosed with high grade dysplasia arising in a hyperplastic polyp. The patient underwent an endoscopy three months later, which showed a fundic gland polyp with low grade dysplasia of the overlying foveolar epithelium. Of note, the patient does not carry mutations associated with familial adenomatous polyposis (FAP) syndrome.
![]()
Table 4: Immunohistochemical profile of squamous morules/mcs.
View Table 4
One patient was deceased approximately five years after the initial endoscopy of unrelated causes. The remaining patients are all alive; no associated malignancies have been reported.
Pathologic impression
Of the four cases received in consultation, one case was not accompanied by an initial diagnosis. Two were submitted with a concern for squamous metaplasia, and as rare epithelial atypia respectively, concerning for carcinoma. One report made no mention of the lesions. These findings appear in table 5.
![]()
Table 5: Initial pathologic impression of squamous morules/mcs.
View Table 5
Discussion
The immunohistochemical and morphological features of gastroesophageal adenoma-microcarcinoids/squamous morules are similar to those previously described in the colorectum. These lesions have been described as 'squamous morules' or 'immature squamous nests' and have been associated with hyperplastic polyps and fundic gland polyps [4]. Our study demonstrates that in addition to displaying a squamoid phenotype (p63), these lesions also label with endocrine markers (synaptophysin and chromogranin). Similar to the colorectal lesions, which all uniformly show nuclear β-catenin labeling; a majority (60%) our cases also showed nuclear positivity regardless of whether the background epithelium was immunoreactive. This finding is common to a number of lesions with a similar morphology found in many neoplastic and reactive processes with APC/beta-catenin pathway alterations [2,5-7].
These lesions show a low proliferative rate as demonstrated by a Ki-67 index of less than 2 percent in all the cases.
Although gastric adenoma-microcarcinoids/squamous morules do not seem to invoke a pseudo-desmoplastic response, they are associated with larger (1.5 cm) polyps with mucosal prolapse type changes. All of the lesions connect with the overlying epithelium, this phenomenon is observed both at the base and the surface of the polyp. Pseudolumina formation is common. Altogether these features are quite easily misinterpreted as dysplastic and in some circumstances as an invasive malignancy (Figure 4). Reporting the presence of these lesions in gastric polyps can help to avoid such pitfalls.
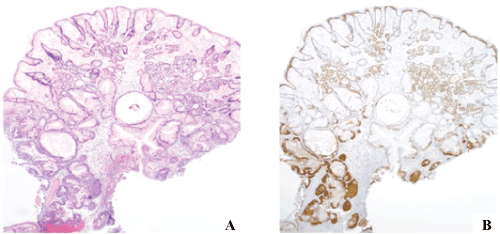
.
Figure 4: Gastroesophageal squamous morules/MC's often arise in large polyps.
(A) They are often multifocal; their connection to the adjacent dysplastic epithelium can easily be misinterpreted as invasion. H&E, 4x. (B) β-catenin, 4x.
View Figure 4
Unlike their colorectal counterparts, which have been encountered almost exclusively in the setting of dysplastic lesions; gastroesophageal adenoma-microcarcinoids/squamous morules were seen in a background of both dysplastic and reactive epithelial changes. Their architecture and proximity to the adjacent epithelium of large polyps often lends itself to over diagnoses of dysplasia and invasive carcinomas. None of the underlying lesions demonstrated any invasive squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma although we have reviewed a limited number of cases [8,9]. Most reported cases, including ours, have shown a benign course [4].
Since the majority of our cases had been received in consultation our study was limited by the amount of follow-up information and the number of unstained slides that we could obtain for immunohistochemical profiling. Although our sample size is small, a search of the literature reveals ours to be among the largest series of adenoma-microcarcinoids/squamous morules.
As for terminology, since these lesions are neither wholly squamous foci nor purely classical neuroendocrine nests, neither term (squamous morule nor adenoma-microcarcinoid) is entirely satisfactory. In our hands, such lesions in the colon all expressed synaptophysin (more sensitive) but not chromogranin (more specific) whereas all lesions expressed either p63 or CK5/6 [2] whereas others found all cases expressed synaptophysin but only 3 of 4 expressed chromogranin and markers of squamous differentiation were not assessed [3]. The pure morphology is more reminiscent of squamous differentiation and borrowing from the gynecologic pathology terminology, namely "squamous morules", seems most satisfactory in describing the pure morphology of these interesting nodules. Furthermore, our concern, especially in gastric samples, is that using "carcinoid" terminology will lead to undue concern on the part of clinical colleagues. Certainly type 1 carcinoids (well-differentiated neuroendocrine tumors) are associated with gastric hyperplastic polyps in patients with autoimmune metaplastic atrophic gastritis [10] but their appearance is that of a classic neuroendocrine lesion. Moreover, gastric neuroendocrine tumors/carcinoids lack any element of squamous differentiation, contrary to the lesions described herein. Similarly, in colorectal pathology, generally true neuroendocrine tumors are a more serious matter and these lesions are essentially incidental findings. We suspect that the high-grade neuroendocrine carcinoma associated with such lesions reported by Lin et al. as case 2 was unrelated to squamous morules. The authors described this lesion as having an infiltrative pattern with involvement of the superficial submucosa [3]. As such, we prefer the old terminology since it underscores the biologic potential of the lesions and better describes their morphology. Although the rate of malignant transformation in hyperplastic polyps is rare 2.1% (range, from 0.8% to 7.1%), it is often demonstrated as glandular dysplasia with or without intestinal metaplasia [11]. These polyps are often large ≥, 1.0 cm, and, frequently demonstrate well to moderately differentiated neoplastic changes.
In summary, awareness of gastroesophageal squamous morules/microcarcinoids and the lesions with which they are associated along with knowledge of their immunohistochemical profile may be helpful in identifying these lesions and thereby avoiding over diagnosing carcinomas. Increased recognition and reporting of these lesions in the pathology report will prevent both misdiagnoses and aggressive treatment regimens.
References
-
Makishi S, Kinjo T, Sawada S, Chinen K, Hirayasu T, et al. (2006) Morules and morule-like features associated with carcinomas in various organs: report with immunohistochemical and molecular studies. J Clin Pathol 59: 95-100.
-
Salaria SN, Abu Alfa AK, Alsaigh NY, Montgomery E, Arnold CA (2013) Composite intestinal adenoma-microcarcinoid clues to diagnosing an under-recognised mimic of invasive adenocarcinoma. J Clin Pathol 66: 302-306.
-
Lin J, Goldblum JR, Bennett AE, Bronner MP, Liu X (2012) Composite intestinal adenoma-microcarcinoid. Am J Surg Pathol 36: 292-295.
-
Schlosnagle DC, Hardin RD (1988) Squamous morules in gastric mucosa. J Clin Gastroenterol 10: 332-334.
-
Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, et al. (2001) Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol 159: 1619-1627.
-
Sekine S, Shibata T, Matsuno Y, Maeshima A, Ishii G, et al. (2003) Beta-catenin mutations in pulmonary blastomas: association with morule formation. J Pathol 200: 214-221.
-
Sekine S, Shibata T, Yamauchi Y, Nakanishi Y, Shimoda T, et al. (2002) Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch 440: 381-386.
-
Mingazzini PL, Barsotti P, Malchiodi Albedi F (1983) Adenosquamous carcinoma of the stomach: histological, histochemical and ultrastructural observations. Histopathology 7:433-443.
-
Straus R, Heschel S, Fortmann DJ (1969) Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer 24: 985-995.
-
Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E (2010) Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol 34: 1591-1598.
-
Imura J, Hayashi S, Ichikawa K, Miwa S, Nakajima T, et al. (2014) Malignant transformation of hyperplastic gastric polyps: An immunohistochemical and pathological study of the changes of neoplastic phenotype Oncol Lett 7: 1459-1463.





