International Journal of Radiology and Imaging Technology
Establishing Radiopharmaceutical Standards at a Nuclear Medicine Unit in Malta
Leonie Pace Vincenti1, Anthony Samuel2 and Francis Zarb3*
1Allied Health Professional (Radiographer), Nuclear Medicine Division, Medical Imaging Department, Mater Dei Hospital, Malta.
2Nuclear Medicine Consultant, Nuclear Medicine Division, Medical Imaging Department, Mater Dei Hospital, Malta.
3Department of Radiography, University of Malta, Malta
*Corresponding author:
Dr. Francis Zarb, PhD, Lecturer, Department of Radiography, Faculty of Health Sciences, University of Malta, Malta, Tel: 00356-23401833, E-mail: francis.zarb@um.edu.mt
Int J Radiol Imaging Technol, IJRIT-2-012, (Volume 2, Issue 2), Original Article
Received: January 28, 2016: Accepted: March 31, 2016: Published: April 02, 2016
Citation: Vincenti LP, Samuel A, Zarb F (2016) Establishing Radiopharmaceutical Standards at a Nuclear Medicine Unit in Malta. Int J Radiol Imaging Technol 2:012
Copyright: © 2016 Vincenti LP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:To establish radiopharmaceutical standards in a Nuclear Medicine Department in terms of radiochemical purity. Radionuclide standards were assessed in terms of radionuclide and chemical purity as well as pH as part of a quality control (QC) programme.
Objectives:An accurate and complete literature search was conducted identifying the needs, specific tests and resources required for the QC programme. A QC protocol was implemented; data collected and findings documented determining current standards and the impact of implementation of a QC programme on work practice.
Methodology:Quantitative data was collected by means of a prospective, non-experimental research design. QC of the radionuclide was performed on all the accessible population, while random sampling was utilized to select a sample to determine the radiochemical purity of radiopharmaceuticals included in the study.
Results:Findings indicate that the radionuclide always met the required standards, which were in accordance with the European Pharmacopeia. The radionuclide purity was up to standards since 99Mo never exceeded more than 0.1% of the total 99mTc activity. The test for chemical purity showed that no samples contained Al3+ and the pH values measured all fell within the accepted range of 4-8. The results however revealed that substandard radiopharmaceuticals were frequently prepared, since the radiochemical purity of several (60.6%) samples fell below the lower limit of acceptance.
Conclusion:The findings indicate that substandard radiopharmaceuticals were a result of preparation error. The results were presented to all staff and work practices are currently being altered to improve radiopharmaceutical standards. The implementation of a full QA programme for radiopharmaceuticals is also in prospect.
Introduction
Radiopharmaceutical preparation and use are regulated by a number of directives, rules and regulations in Europe, as they are considered to be a special group of medicines [1]. A quality control (QC) program is essential for all hospital departments dealing with the preparation of radiopharmaceuticals [2].
The aim of this research study was to implement a QC protocol to establish the standards of radiopharmaceuticals administered to patients in the Nuclear Medicine Department at the public general hospital in Malta.
Literature Review
In conventional Nuclear Medicine it is of great importance that all materials and products administered into the human body, including 99mTc radiopharmaceutical kits, are safe and of constant high quality in producing the required effects [3]. The quality of a product may be described as the extent of product compliance to a predetermined set of standards or requirements [4]. Manufactures have an obligation to make certain that all released products are suitable for their intended use. All pharmaceuticals must conform to specifications that assure products are of suitable quality and are adequate and safe for patient administration [5]. This also applies to radiopharmaceuticals since, like pharmaceuticals, these products are administered to humans [6].
Quality Assurance (QA) ensures consistent good quality service and involves several steps and adherence to pre planned procedures, contributing to achieving the highest quality for the final products intended use [7,8]. When it comes to the production of radiopharmaceuticals, QA has a vital role in ensuring that the end product is of sufficient quality. This is especially true in certain circumstances when, due to certain characteristics of radiopharmaceuticals, it is not possible to carry out all the necessary quality control tests prior to releasing the radiopharmaceutical for human administration or animal administration, in case of preclinical studies. QA may therefore reduce the possibilities of substandard products being produced by adhering to procedures and protocols that will collectively contribute to the production of radiopharmaceuticals that adhere with specifications [9,10].
Quality Control (QC) is an essential part of QA and is necessary to ensure an adequate QA programme to protect patients from unnecessary ionising radiation [11]. QC is the part of a QA programme that assesses whether the performance level required of the service has been reached by documenting measurements obtained from tests and checks performed in various areas in nuclear medicine, such as all equipment and radiopharmaceuticals. The results obtained from QC may then be compared to pre set standards to make certain that the results are within the established limits. QC may therefore certify that a product is of good quality and fit for its intended use [8,11].
According to the European Association of Nuclear Medicine (EANM), the quality of all radiopharmaceutical products should be determined prior to release for human administration and a QC programme should be in place at any hospital department that deals with radiopharmaceutical kit preparation [11]. Although most of the responsibility of radiopharmaceutical quality belongs to the manufacturer of the pharmaceutical, the nuclear medicine department producing the final product for human administration (radiopharmaceutical) also has the responsibility of ensuring that the radiopharmaceutical produced is up to standard [12-14].
QC tests are performed on the pharmaceutical kits by the manufacturer prior to them being released for sale, therefore the manufacturer is guaranteeing that the kits are up to standard [10]. Once the radionuclide is added to the pharmaceutical kit, a complex chemical reaction takes place and it is imperative to determine whether the chemical reaction has taken place and that the radiopharmaceuticals are suitable for patient administration [15]. Impurities present in the resultant radiopharmaceutical may alter bio-distribution within the patient, which may have an effect on image quality; cause unnecessary irradiation of organs and in some cases may possibly result in a misdiagnosis [4].
Quality Control of Radionuclides and Radiopharmaceuticals
Callahan et al., (2007), provide guidelines for the elution of generators and the preparation of kits on-site and mention the various parameters that should be tested in a QC programme [15]. An extensive QC programme should be in place to monitor these parameters. It should be taken into consideration that practices may vary tremendously within different departments and between countries and it may not always be possible to monitor all these recommended parameters in a QC programme.
According to Early & Sodee, (1995) the radionuclide purity and the radiochemical purity are two areas that are of utmost importance when determining the quality of the radiopharmaceutical. Therefore these two parameters should always be included in a basic QC programme if it is not possible to set up a comprehensive QC programme [12].
Radionuclide purity
Radionuclide purity may be defined as that percentage of radioactivity that makes up the specified radionuclide. The purity of the radionuclide is based upon the percentage of the radionuclide present in the desired radionuclide (free from contaminants) and determines whether other radionuclides that are not of interest are present in the solution. Any other radionuclide other than the one of interest is considered to be an impurity [16].
Chemical purity
Chemical purity refers to the amount of the desired chemical in the radiopharmaceutical. Determining the chemical purity of the radionuclide allows identification of any undesired chemicals in the eluate [17].
pH of the eluate
The pH is a measure of hydrogen ions in a given solution and is used as a measure on aqueous solutions to determine their acidity or alkalinity [18].
Radiochemical purity
The radiochemical purity of radiopharmaceuticals is the fraction of radionuclide found in the desired form, that is the percentage of the radionuclide that has bound to the ligand in the kit, and the fraction of radionuclide that did not bind to the ligand and remained 'free' in the solution [4,11,19].
Once the necessary QC tests have been performed on the eluate and it is guaranteed that the eluate is free from contaminants and impurities, that could potentially affect the quality of the final product (the radiopharmaceutical), the radionuclide, in the form of sodium pertechnetate is added to the kit to produce the required radiopharmaceutical [19].
Methods
In this study, quality standards of radiopharmaceuticals were determined through the implementation of a QC protocol whereby QC tests were performed and data collected. The research design was a quantitative, prospective, non-experimental evaluation of radiopharmaceuticals within a general public hospital in Malta.
QC tests were identified after a review of relevant literature. Tests were performed on the 99mTc eluates identified in accordance with the European Pharmacopeia as well as available resources and included: radionuclide purity, chemical purity and pH. The radiochemical purity of reconstituted radiopharmaceuticals was also determined.
QC tests were randomly performed on the two most commonly used radiopharmaceuticals. Table 1 provides a summary of: the sample tested; the QC procedure; test performed; measuring instrument and what was being measured for each of the tests.
![]()
Table 1: Summary of QC tests.
View Table 1
Results
Radionuclide purity
A total of 94 eluate samples were tested for radionuclide purity over a 2 month period. The results showed that 99Mo is eluted together with 99mTc from the generator column on rare occasions and only 10 (10.6%) samples were found to have contamination. However the amount of 99Mo never exceeded the accepted limit since the amount of 99Mo present was never greater than 0.1% of the eluted 99mTc.
Chemical purity
All 94 eluate samples (100%) did not exceed the accepted limits of 10 μg/ml of Al3+ within the eluate.
pH
The pH measurements ranged between 5-7, with values similar to those obtained in other studies that assessed the quality of 99mTc eluates in terms of pH [20,21]. The pH of all eluates was within the accepted range of 4-8, as recommended by the European Pharmacopeia as cited by Zolle, (2007) [22]. In all instances the pH value obtained on the first elution was the same value measured for subsequent elutions for that particular generator and that pH values only varied between generators.
Radiochemical purity
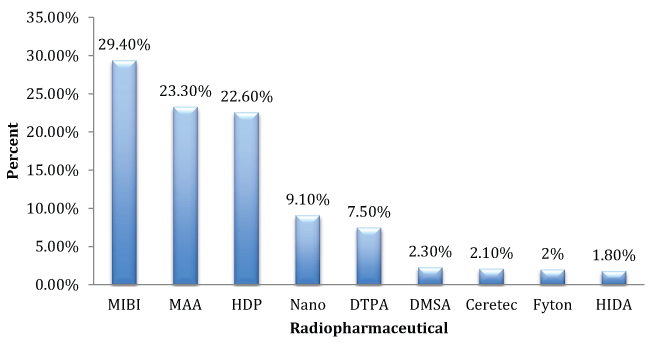
Figure 1 provides the frequency of reconstitution of the 9 99mTc radiopharmaceuticals available in the local nuclear medicine department. The second most frequently used radiopharmaceutical was Macro aggregated albumin (MAA), however the radiochemical purity of MAA could not be determined with chromatography methods alone [23] and due to lack of resources MAA was excluded from the study. All other radiopharmaceuticals formed less than 10% of kits reconstituted and QC was not performed on these radiopharmaceuticals since not enough data would have been collected during the data collection period.
Therefore, the two most commonly used radiopharmaceuticals identified on which the QC tests could be performed were:
• Methoxyisobutylisonitrile (MIBI), and
• Hydroxymethylene diphosphonate (HDP)
QC to determine the radiochemical purity of MIBI and HDP was performed on all kits reconstituted during the data collection period. A total sample size of 33 was obtained, of which 17 samples were MIBI radiopharmaceuticals and the other 16 samples were HDP radiopharmaceuticals. Substandard radiochemical purities amounted to 60.6% while the remaining 39.4% had acceptable levels of radiochemical purities. These results demonstrate that more than half of the samples tested did not meet the required standards. The results also showed that the majority (51.5%) of the samples that were not up to standards were MIBI radiopharmaceuticals.
MIBI radiopharmaceuticals should have a radiochemical purity ≥ 94%. None of the results of MIBI radiopharmaceuticals tested was greater than 94%. This indicates that all (100%) MIBI radiopharmaceuticals were substandard (Mean: 86.76%; Min: 75%; Max: 93%)
HDP radiopharmaceuticals should have a radiochemical purity ≥ 90%. The majority (81.3%) of HDP radiopharmaceuticals analysed had a radiochemical purity > 90%. Out of the 16 samples tested, only 3 (18.7%) had a radiochemical purity below the accepted limit (Mean: 92.86%; Min: 87%; Max: 97.3%).
The one-sample t-test was used to compare the sample mean proportion of the radiochemical purity obtained for each radiopharmaceutical, with the lower limit of acceptance (90% for HDP and 94% for MIBI) (Table 2).
![]()
Table 2: One-Sample T-Test - MIBI (Lower Limit of 95%).
View Table 2
The mean sample proportion of radiochemical purity when using MIBI (86.76%) was found to be significantly lower than the lower limit of acceptance (94%), since the p value (p < 0.001) is < the 0.05 level of significance (Table 2).
The mean sample proportion of radiochemical purity when using HDP (92.86%) was found to be significantly higher than the lower limit of acceptance (90%), since the p-value (p = 0.002) is < the 0.05 level of significance (Table 3), indicating that there is a significant difference between the mean radiochemical purity and the 90% standard. Since the mean is higher than the standard, it indicates that only on rare events are substandard HDP radiopharmaceutical kits prepared and the majority of all HDP kits are well above the 90% standard.
![]()
Table 3: One-Sample T-Test - HDP (Lower Limit of 90%).
View Table 3
Discussion
Findings show that 99mTc elutions always met required standards in relation to the radionuclide and chemical purity, as well as the pH of the elutions. Rarely were any contaminants present in the elution. Even on those occasions were 99Mo contaminants were discovered in the elution, the amount always fell well within the accepted limit and had no impact on quality. It is still stressed however that routine QC is performed to ensure that limits are never exceeded and provide a high quality service to patients.
Findings related to the quality of radiopharmaceuticals revealed that a large number (60.6%) of samples tested did not meet the required standards of radiochemical purity and it is necessary to improve the quality of injected radiopharmaceuticals, especially when preparing MIBI. In view of these results, it is evident that impurities were a result of preparation issues and all radiographers involved in the preparation of radiopharmaceuticals must adhere to preparation instructions to reduce the amount of impurities.
Corrective measures to increase the radiochemical purity of MIBI radiopharmaceuticals are currently underway. The researcher highlighted the factors that could possibly interfere with the reconstitution of radiopharmaceuticals. These factors include: the heating time, volume, the introduction of air into the vial during preparation, the amount of 99mTc added to the kit and the age of the 99mTc eluate used. A great emphasis has been made on the importance of adhering to the preparation instructions, since deviating from the preparation instructions provided in the MIBI package insert could be a potential cause of the substandard MIBI kits being prepared.
Frequent testing of MIBI preparations are being carried out to bring the product up to standards and determine whether altering work practice will improve the quality. The department is also ordering 99Mo/99mTc generators of a higher specific activity to adhere to the volume specified in the preparation instructions. Substandard samples of HDP occur less frequently however they still occur, therefore frequent testing is also recommended.
As with all research there were limitations to the study. Due to lack of resources and the expenditure required as well as the longer data collection period necessary not all radiopharmaceuticals were tested. However a protocol was developed which could easily be updated to include all radiopharmaceuticals when implementing a full QA program once the necessary resources are made available. The research study was a learning experience, not just for the researcher, but also for colleague radiographers involved in the data collection.
Conclusion
The results of this study provide a review of the quality standards of radionuclides and radiopharmaceuticals in a nuclear medicine department in Malta. The study provided awareness of the current local situation by presenting the findings and recommending work practice improvements and further research to better the departmental quality and service.
Routine QC tests are recommended to improve the quality of the elute even though contaminants are rarely present.
To improve the quality of radiopharmaceuticals associated with radiochemical purity, radiopharmaceutical preparation should adhere with preparation instructions. A study to determine the causes of the increased amount of impurities present with the aim of eliminating them and producing products of higher purity is recommended. This study provided a baseline for comparison of future research findings.
There were few studies directly related to the investigation of radiopharmaceutical quality and literature is mainly limited to QC and the importance of QC. This signifies the importance of this research study since the results obtained would contribute to the current available body of knowledge on the topic.
References
-
Elsinga P, Todde S, Penuelas I, Meyer G, Farstad, B, et al. (2010) Guidance on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. Eur J Nucl Med Mol Imaging 37: 1049-1062.
-
UK Radiopharmacy Group; NHS Pharmaceutical Quality Control Committee (2001) Quality assurance of radiopharmaceuticals. Report of a joint working party: the UK Radiopharmacy Group and the NHS Pharmaceutical Quality Control Committee. Nucl Med Commun 22: 909-916.
-
Verbruggen A, Coenen HH, Deverre JR, Guilloteau D, Langstrom B, et al. (2008) Guideline to regulations for radiopharmaceuticals in early phase clinical trials in the EU. Eur J Nucl Med Mol Imaging 35: 2144-2151.
-
Sharp PF, Gemmell HG, Murray AD (2005) Practical Nuclear Medicine. Springer, London.
-
Haleem RM, Salem MY, Fatahallah FA, Abdelfattah LE (2015) Quality in the pharmaceutical industry - A literature review. Saudi Pharm J 23: 463-469.
-
Saha GB (2010) Fundamentals of Nuclear Pharmacy (6th Edition ed.) Springer Science + Business Media, New York, USA.
-
Owen F, Maidment D (1996) Quality Assurance: A Guide to the Application of ISO 9001 to Process Plant Projects (2nd Edition ed.) Rugby, Institution of Chemical Engineers, Warwickshire.
-
Khurshid SJ, Sadiq MZ (1996) Quality Assurance in Nuclear Medicine - Biological Quality Control of Radiopharmaceuticals. Pakistan Journal of Pharmaceutical Sciences 9: 43-54.
-
European Commission (2008) EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal Products (corrected version). EudraLex The Rules Governing Medicinal Products in the European Union.
-
EANM (2007) Guidelines on Current Good Radiopharmacy Practice (cGrPP) in The Preparation of Radiopharmaceuticals, Version 2, European Association of Nuclear Medicine.
-
EANM (2008) The Radiopharmacy: A Technologist's Guide. Vienna: European Association of Nuclear Medicine.
-
Early PJ, Sodee DB (1995) Principles and Practice of Nuclear Medicine (2nd Edition). St.Louis, Mosby.
-
Wang YF, Chuang MH, Chiu JS, Cham TM, Chung MI (2007) On-site preparation of technetium-99m labeled human serum albumin for clinical application. Tohoku J Exp Med 211: 379-385.
-
Hung JC (2011) Quality Control in Nuclear Pharmacy. In: RJ Kowalsky, SW Falen, Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine (3rd Edition). American Pharmacists Association, Washington p: 273.
-
Callahan RJ, Chilton HM, Ponto JA, Swanson DP, Royal HD, et al. (2007) Procedure guideline for the use of radiopharmaceuticals 4.0. J Nucl Med Technol 35: 272-275.
-
Hoory S, Bandyopadhyay D, Vaugeois JC, Levy LM (1986) Impurities in generator eluates and radiopharmaceuticals: a computerized quality assurance approach. Health Phys 50: 843-848.
-
Hamilton D (2004) Diagnostic Nuclear Medicine: A Physics Perspective. Springer, Berlin.
-
Prichard E (2003) Practical Laboratory Skills Training Guide: Measurement of pH. The Royal Society of Chemistry, Cambridge, UK.
-
Mettler FA, Guiberteau MJ (2012) Essentials of Nuclear Medicine Imaging (6th Edition) Philadelphia: Elsevier Health Sciences.
-
Hammermaier A, Reich E, Bögl W (1986) Chemical, radiochemical, and radionuclide purity of eluates from different commercial fission 99Mo/99mTc generators. Eur J Nucl Med 12: 41-46.
-
Andrade WG, Lima FF (2009) Evaluation of the eluate quality of 99Mo-99mTc generators in Recife, International Nuclear Atlantic Conference, Brazil.
-
Zolle I (2007) Technetium-99m pharmaceuticals: Preparation and quality control in nuclear medicine. Springer, Heidelberg, Berlin.
-
Owunwanne A, Patel M, Sadek S (1995) The handbook of Radiopharmaceuticals. England: Chapman & Hall Medical, London.